Abstract
Aims: We present a 5-case series of low-grade oncocytic tumour of the kidney to further discuss their clinicopathological characteristics.
Methods and results: Five patients were included in this study. There were three females and two males aged 45–66 years, with a median age of 65 years. Four tumours were located in the right kidney, and one was located in the left kidney. Most of the tumour sections were yellow-brown in colour. Tumour sizes ranged from 2.5 to 4.5 cm, with a median size of 3 cm. Microscopically, the tumours were well-circumscribed but lacked a fibrous capsule; the tumours consisted of monomorphous oncocytic cells arranged mainly in solid and nested architectural patterns. The tumour cells had uniformly round to oval nuclei and often had perinuclear halos but lacked significant irregularities. Immunohistochemically, the tumour cells showed a diffuse and strong positivity for CK7 and were negative for CD117. The tumour cells were also positive for GATA3, E-cadherin, Pax-8, Succinate dehydrogenase B (SDHB) and Fumarate hydratase (FH), and negative for vimentin, Carbonic anhydrase 9 (CA9), CD10, P504s, CK20, TFE3, TFEB, HMB45, ALK and Forkhead box protein I1 (FOXI1). Next-generation sequencing identified genetic variations in these tumours, including MTOR gene mutations (4/5) and PIK3CA gene mutation (1/5). All patients were alive without disease progression at a median follow-up of 32 months (range 10–57 months).
Conclusion: LOT is an emerging renal entity of indolent behaviour that has morphologic overlap with some renal tumours with eosinophilic cytoplasm, primarily with oncocytoma and eosinophilic variant of chromophobe renal cell carcinoma. Familiarity with the distinctive morphological features, immunophenotype and molecular genetics of LOT helps avoid misdiagnosis.
Introduction
Low-grade oncocytic tumour (LOT) of the kidney is an emerging renal entity that was first described by Trpkov K and Hes O in 2019 (1). This low-grade entity is composed of oncocytic tumour cells, and characterized by a CK7-positive/CD117-negative immunoprofile (2). Several case series have been reported. In the current study, we present five patients with LOT, and the clinical, histological, immunophenotypic and molecular features were analysed. The aim of our study was to further discuss the clinicopathological features and differential diagnosis of LOT.
Materials and methods
Case selection
From 2017 to 2021, 876 renal tumours were processed in The Third Affiliated Hospital of Soochow University/Changzhou First People’s Hospital. A series of 62 oncocytic renal tumours were identified, including 19 cases of eosinophilic variant of chromophobe renal cell carcinoma (E-ChRCC), 13 cases of renal oncocytoma, 16 cases of papillary renal cell carcinoma (PRCC) with eosinophilic features, 8 cases of clear cell renal cell carcinoma (CCRCC) with eosinophilic features, and 6 cases of unclassified oncocytic renal tumour. We only identified 5 tumours as LOT characterized by CK7 positivity and CD117 negativity. Of the five cases, four were originally diagnosed as E-ChRCC, and one as renal oncocytoma. The clinical and pathological findings were obtained from the medical records and pathology reports. Follow-up information was obtained by direct telephone communication with the patients and/or their relatives.
Immunohistochemical analysis
Immunohistochemical staining was performed on 4-µm-thick formalin-fixed, paraffin-embedded (FFPE) tissue sections using a Roche Benchmark XT Automated Staining System. The antibodies used in this study (Supplementary Table S1) included CK7 (EP16, prediluted, ZSGB-BIO), CD117 (YR145, prediluted, MXB Biotechnologies), Carbonic anhydrase 9 (CA9) (H-11, prediluted, ZSGB-BIO), CD10 (MX002, prediluted, MXB Biotechnologies), P504s (13H4, prediluted, ZSGB-BIO), Pax-8 (EP298, prediluted, MXB Biotechnologies), vimentin (UMAB159, prediluted, ZSGB-BIO), E-cadherin (MX020, prediluted, MXB Biotechnologies), HMB45 (HMB45, prediluted, MXB Biotechnologies), CK20 (EP23, prediluted, ZSGB-BIO), Succinate dehydrogenase B(SDHB) (OTI1H6, prediluted, ZSGB-BIO), Fumarate hydratase(FH) (OTI1F10, prediluted, ZSGB-BIO), ALK (5A4, prediluted, MXB Biotechnologies), TFE3 (EP285, prediluted, ZSGB-BIO), TFEB (OTI2C1, 1:500, OriGene), GATA3 (EP368, prediluted, ZSGB-BIO), Forkhead box protein I1 (FOXI1) (EPR22940-151, 1:100, Abcam) and Ki-67 (UMAB107, prediluted, ZSGB-BIO). Positive and negative controls were used for each antibody. Immunoreactivity was scored by the percentage of positive tumour cells as follows: <1% (negative), 1%–50% (focal positive), and > 50% (diffusely positive).
Next-generation sequencing
Next-generation sequencing (NGS) was performed by Illumina MiSeq (Illumina). The tissue DNA was extracted from FFPE tumour tissues using QIAamp DNA FFPE tissue kit (Qiagen, Hilden, Germany). Fragments between 200 and 400 bp from the sheared tissue DNA were purified (Agencourt AMPure XP Kit, Beckman Coulter, CA, United States), hybridized with capture probes baits, selected with magnetic beads, and amplified. Target capture was performed using a commercial panel consisting of 520 genes (Supplementary Table S2) chosen by Guangzhou Burning Rock Biotech Ltd. Sequence data were mapped to the reference human genome (hg19) using Burrows-Wheeler Aligner version 0.7.10. Local alignment optimization, duplication marking and variant calling were performed using Genome Analysis Tool Kit version 3.2, and VarScan version 2.4.3. Base calling in tissue samples required at least 8 supporting reads for single nucleotide variations (SNVs) and 2 and 5 supporting reads for insertion-deletion variations (Indels), respectively. Copy number variations (CNVs) were analyzed based on the depth of coverage data of capture intervals.
Results
Clinical features
The clinical features of the five patients are summarized in Table 1. There were three females and two males with ages ranging from 45 to 66 years (mean, 59 years; median, 65 years). All tumours were detected during a physical examination. Three patients underwent partial nephrectomy, and two patients underwent radical nephrectomy. Four tumours were located in the right kidney, and one was located in the left kidney. The tumour’s cut surface was mostly yellow‒brown in colour. Tumour size ranged from 2.5 to 4.5 cm in the maximal diameter (mean, 3.3 cm; median, 3 cm). No adjunctive treatment was administered. All patients had no evidence of local recurrence or distant metastasis during follow-up, which ranged from 10 to 57 months (mean, 34 months; median, 32 months).
TABLE 1
| Case | Age | Sex | Site | Size (mm) | Type of surgery | Status | Follow up (months) |
|---|---|---|---|---|---|---|---|
| 1 | 45 | Female | Right | 25 | Partial | Alive no evidence of disease | 57 |
| 2 | 65 | Male | Right | 30 | Partial | Alive no evidence of disease | 53 |
| 3 | 66 | Female | Right | 35 | Radical | Alive no evidence of disease | 32 |
| 4 | 56 | Male | Left | 45 | Partial | Alive no evidence of disease | 21 |
| 5 | 65 | Female | Right | 30 | Radical | Alive no evidence of disease | 10 |
Clinicopathological features of low-grade oncocytic tumour (LOT) of the kidney.
Pathological features
Histologically, all tumours were well circumscribed but lacked a fibrous capsule. The growth patterns included solid, nested (Figure 1), trabecular and microcystic growth (Figure 2). There were abundant thin-walled vessels around the tumour cell nest (Figure 1). Lymphocytic clusters (Figure 1) were observed in one tumour (Case 3). One tumour (Case 4) exhibited thick-walled vessels. Oedematous stromal areas were seen in two tumours (Case 2 and Case 4), with scattered single-cell arrangement in these areas (Figure 3). Focal fresh haemorrhage was also noted in three tumours (Cases 2, 3 and 4). No necrosis was observed in any of the tumours. The tumour cells had indistinct cell membranes, monomorphous oncocytic cytoplasm, and uniformly round to oval nuclei without mitotic activity. The tumour cells had perinuclear halos (Figure 1) but lacked significant irregularities. The nucleoli were slightly conspicuous (Figure 1).
FIGURE 1
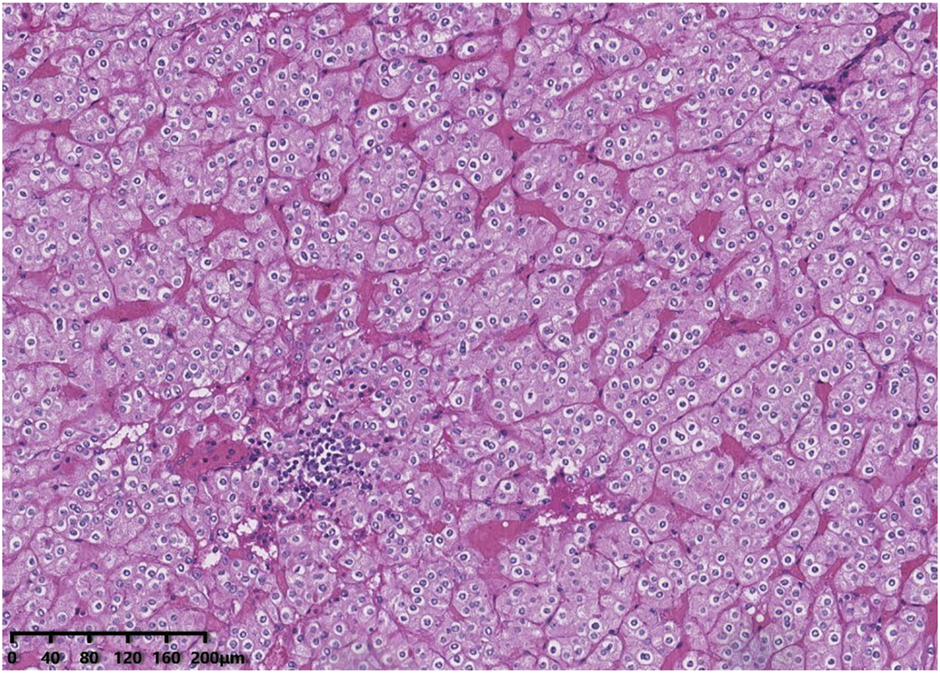
Histological features of low-grade oncocytic tumour (LOT) of the kidney. The tumour cells demonstrated a nested growth pattern. There are abundant thin-walled vessels around the tumour cell nest. Lymphocytic clusters were seen in this area. The tumour cells had perinuclear halos but lacked significant irregularities. The nucleoli were slightly conspicuous.
FIGURE 2
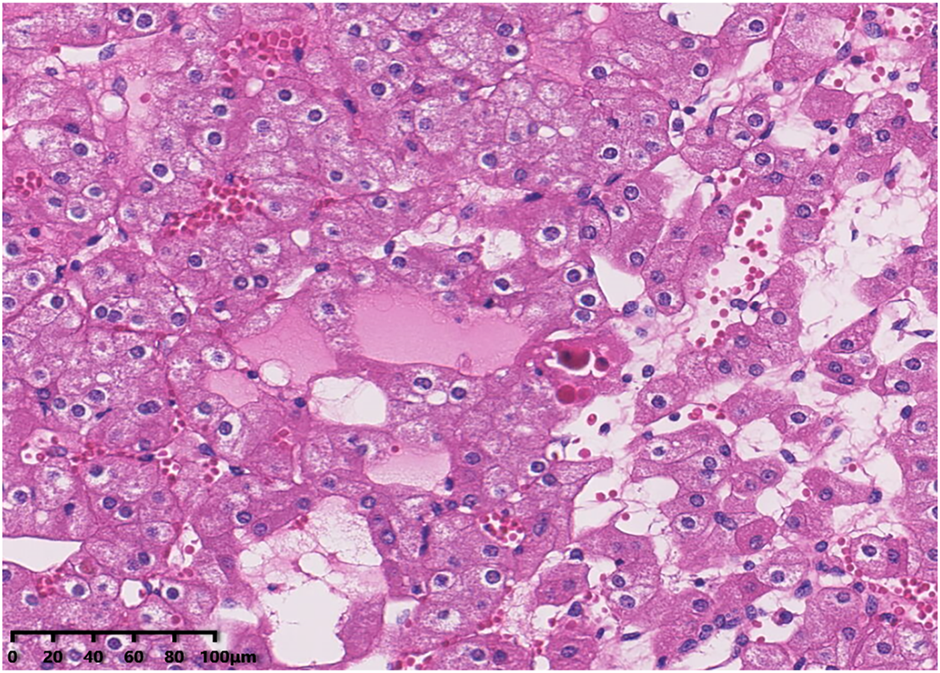
The tumour cells demonstrated a microcytic growth pattern.
FIGURE 3
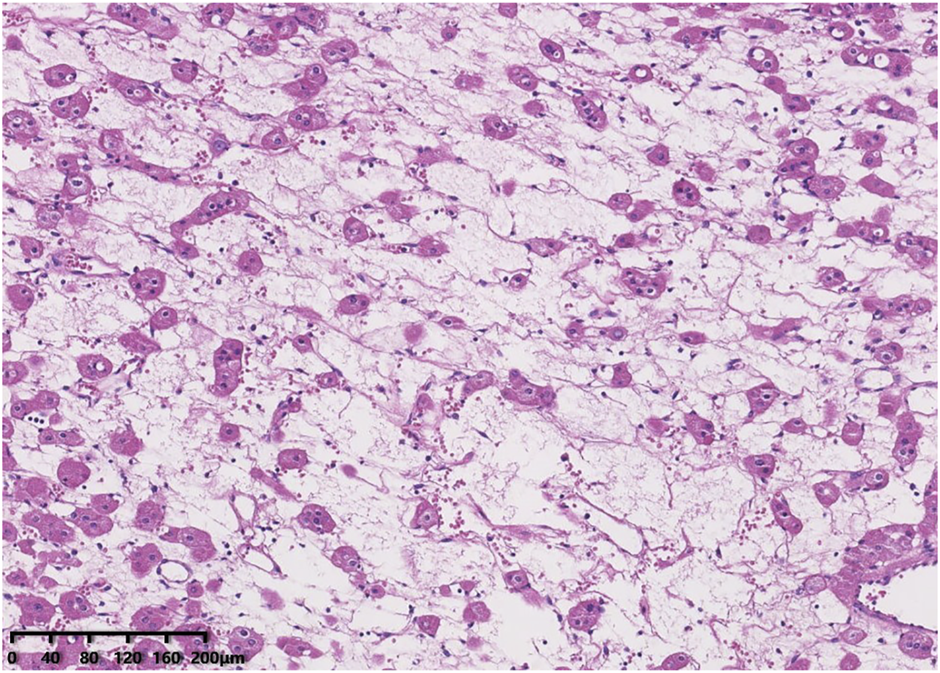
Oedematous stromal areas are presented, with scattered single-cell arrangement in these areas.
Immunohistochemically, all tumours showed diffuse and strong positivity for CK7 and were negative for CD117. GATA binding protein 3 (GATA3) (Figure 4), E-cadherin and Pax-8 were also present in all neoplasms. SDHB and FH were retained. All tumour cells were negative for vimentin, CA9, CD10, P504s, CK20, TFE3, TFEB, HMB45, ALK and FOXI1. The Ki-67 index was less than 3%. NGS identified genetic variations in these tumours (Table 2). Four tumours had a MTOR gene exon 53 p.L2427Q mutation [c.7280T>A (p.Leu2427Gln)], and one tumour had a PIK3CA gene exon 10 p.E542K mutation [c.1624G>A (p.Glu542Lys)].
FIGURE 4
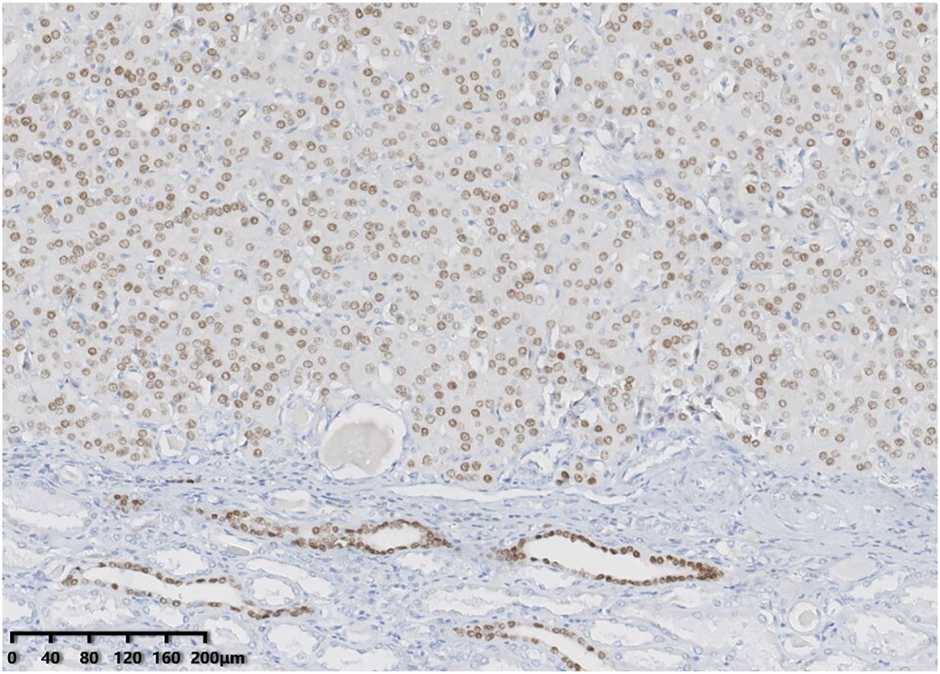
GATA3 showed consistent positivity in low-grade oncocytic tumour (LOT) of the kidney.
TABLE 2
| Case | Gene | Mutation_type | Description | Allele fraction (%) | Depth | Exon_number | Chromosome | Position | Hgvs_c | Hgvs_p |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PRKDC | synonymous_variant | p.Y2483= | 43.70 | 1389 | 55 | 8 | 48752579 | c.7449C>T | p.Tyr2483= |
| APC | stop_gained | p.R283* | 17.26 | 2132 | 9 | 5 | 112151204 | c.847C>T | p.Arg283* | |
| KDR | missense_variant | p.I519V | 47.06 | 1719 | 12 | 4 | 55972089 | c.1555A>G | p.Ile519Val | |
| EP300 | missense_variant | p.P210S | 45.35 | 3063 | 2 | 22 | 41513724 | c.628C>T | p.Pro210Ser | |
| MTOR | missense_variant | p.L2427Q | 20.92 | 2510 | 53 | 1 | 11174395 | c.7280T>A | p.Leu2427Gln | |
| SPEN | missense_variant | p.D303V | 47.95 | 1293 | 4 | 1 | 16235842 | c.908A>T | p.Asp303Val | |
| 2 | MTOR | missense_variant | p.L2427Q | 9.34 | 1413 | 53 | 1 | 11174395 | c.7280T>A | p.Leu2427Gln |
| 3 | MTOR | missense_variant | p.L2427Q | 18.62 | 1847 | 53 | 1 | 11174395 | c.7280T>A | p.Leu2427Gln |
| 4 | PIK3CA | missense_variant | p.E542K | 24.85 | 1356 | 10 | 3 | 178936082 | c.1624G>A | p.Glu542Lys |
| CDK4 | synonymous_variant | p.A16= | 35.69 | 1303 | 2 | 12 | 58145453 | c.48C>T | p.Ala16= | |
| 5 | PDGFRB | synonymous_variant | p.V480= | 44.71 | 2098 | 10 | 5 | 149509459 | c.1440G>A | p.Val480= |
| MTOR | missense_variant | p.L2427Q | 17.73 | 1743 | 53 | 1 | 11174395 | c.7280T>A | p.Leu2427Gln | |
| JAK3 | splice_acceptor_variant | c.1787-2A>G | 52.48 | 747 | 14 | 19 | 17946862 | c.1787-2A>G | ||
| FAT1 | intron_variant | c.13139-7C>T | 43.38 | 989 | 27 | 4 | 187510381 | c.13139-7C>T |
Genetic variations in the five adults with low-grade oncocytic tumour (LOT) of the kidney.
Abbreviations: Hgvs, human genome variation society.
Discussion
Low-grade oncocytic tumour (LOT) of the kidney has emerged as a new diagnostic entity in renal tumour pathology in recent years (2–13). LOT is now enrolled in a new tumour subgroup called other oncocytic tumours of the kidney in the fifth edition of the WHO classification of urinary and male genital tumours (14), and is defined as a neoplasm with bland low-grade nuclei, diffuse strong CK7 labelling, and negative CD117 labelling. From 2017 to 2021, the incidence rates of LOT in our hospital were approximately 0.57% among renal tumours and 8% among oncocytic tumours in our study, while the incidence rates reported previously were 0.18% (4), 0.35% (5) and 0.17% (7) among renal cell tumours, respectively.
The clinicopathological features of LOT in this study are highly consistent with those of previously published cases. The tumours are found accidentally during physical examination in older patients as a single tumour, have a slight female predilection and have an indolent behaviour. Several LOTs have been reported in patients with tuberous sclerosis complex (TSC) (5, 6, 8) or end-stage renal disease (ESRD) (5). Multiple and/or bilateral renal tumours have been discovered (5, 6). Individual patients have died from unrelated diseases (5, 8, 12). The neoplasms in this study were typically well-circumscribed solid tumours consisting of monomorphous oncocytic cells arranged mainly in solid and nested architectural patterns, with no papillary growth pattern. The tumour cells were monomorphous with indistinct cell membranes and had an eosinophilic cytoplasm and prominent round to oval nuclei that lacked significant irregularities and often had perinuclear halos. Cystic change and central scarring are only seen in larger tumours (3) in general. Individual neoplasms have perirenal fat infiltration (3). Renal tubules can be entrapped at the periphery in individual tumours (4). Spindle elongated tumour cells are also observed, especially in hypocellular areas with oedematous stroma, such as cell culture growth. Muller–Mowry colloidal iron staining of LOT was either negative or only luminally positive (2, 3).
All the five tumours in this study had a typical immunophenotype characterized by diffuse positivity for CK7 and negativity for CD117, and were also positive for PAX-8, E-cadherin, FH and SDHB, but negative for vimentin, CD10, P504s, CA9, CK20, TFE3, TFEB, HMB45, and ALK. Rare neoplasms can show weak focal CD117 staining (2). Vimentin, CD10 and P504s can be focally positive (3, 4). BerEP4 (2, 4), MOC31 (2), CyclinD1 (4), 4EBP1 and S6K (8, 9) are also positive in LOT. Morini et al. (9) found no expression of FOXI1 in LOT. FOXI1 is a member of the forkhead transcription factor family, and high expression of FOXI1 has been found in restricted normal cell types, such as renal intercalated cells (ICs). Research has shown that FOXI1 is a potential biomarker of IC-related renal tumours, such as ChRCC and renal oncocytoma (15). We also identified negativity for FOXI1 in the current study. GATA3 belongs to the family of transcription factors that recognizes G-A-T-A nucleotide sequences in the target gene and is mostly used as a marker for breast and urothelial carcinomas. Studies (16) have found that GATA3 is expressed in distal nephrons, 51% of ChRCCs and 17% of oncocytomas. GATA3-positive reactions have also been documented in clear cell papillary renal cell tumour (17) and the recently recognized papillary renal neoplasm with reverse polarity (18). Therefore, GATA3 is not an entirely specific marker for one entity of renal cell neoplasms. Researches (12, 19) recently noted consistent GATA3 immunohistochemical positivity in LOT. We also observed GATA3 positivity in this study. Therefore, it can be inferred that the findings of FOXI1 negativity and consistent GATA3 positivity further expand the expected pattern of immunohistochemical markers in LOT.
Reports on the genetic analysis and molecular pathology of LOT are limited. Trpkov et al. (2) initially found that there were deletions at 19p13.3, 1p36.33 and 19q13.11 in some LOT patients by using array comparative genomic hybridization (ACGH). CCND1 rearrangements were not found in LOT by using fluorescence in situ hybridization (FISH) (5). An increasing number of genetic tests have identified genetic mutations in LOT patients, including RHEB (8), MTOR (7–13), TSC1 (6–9, 11–13), TSC2 (11, 12), and PIK3CA (12), which are primarily involved in the mTOR pathway. The PI3K/AKT/mTOR signalling pathway plays a key role in cell survival and growth, mTOR is the master regulator of cell metabolism and growth, and acts through two different multiprotein complexes, namely, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 is regulated by the tuberous sclerosis complex (TSC) (12). 4EBP1 and S6K expressed in LOT are the main downstream effectors of mTORC1 (20). All of these results confirm that LOT is a mTOR pathway mutation-associated renal tumour. We also found MTOR gene mutations in four tumours and an uncommon PIK3CA gene mutation in one tumour in this study.
Pivovarcikova et al. (21) discussed three TSC/mTOR pathway mutation-associated eosinophilic renal tumours, including eosinophilic solid and cystic renal cell carcinoma (ESC RCC), eosinophilic vacuolated tumours (EVT) and LOT. ESC RCC (22) is arranged in solid areas of eosinophilic cells with cytoplasmic stippling combined with cystic spaces lined by similar cells with a hobnail-shaped configuration. The tumour typically demonstrates the CK7-/CD117-/CK20+/vimentin+ immunophenotype. EVT demonstrates a solid growth architecture, large eosinophilic cells with distinct intracytoplasmic vacuoles, prominent cell membranes, large nuclei with prominent nucleoli, a CK7-/CD117+/CD10+/cathepsin K+ immunophenotype (23), and sporadic TSC/MTOR mutations (24). Recently, Xia et al. (13) further explored the molecular characteristics of TSC/MTOR-associated eosinophilic renal tumours, which included ESC RCC, EVT, LOT and unclassified renal tumours with TSC/MTOR mutations (TSC-mt RCC-NOS). They observed a specific trend in TSC/MTOR mutation in different tumours. ESC RCC and TSC-mt RCC-NOS displayed consistent TSC1/TSC2 mutations, EVT demonstrated equal mutation distributions in TSC and MTOR genes, and all LOT cases but one bearing TSC1 mutation displayed MTOR gene mutations.
LOT shows some similarities with oncocytoma and E-ChRCC. Oncocytoma often contains the CD117+/CK7− immunophenotype, and commonly exhibits recurrent chromosomal losses (1, 14, 21, X, Y) (14). E-ChRCC is characterized by eosinophilic tumour cells with raisinoid nuclei and perinuclear haloes and demonstrates CK7+/CD117+ immunophenotype. Some tumours are associated with Birt-Hogg-Dubé (BHD) syndrome harbouring FLCN germline mutations. Hybrid oncocytic/chromophobe tumour (HOCT) was initially described in patients with BHD syndrome harbouring FLCN gene mutations (14). The other differential entities include SDH-deficient RCC, TFEB translocation RCC, and oncocytic type of FH-deficient RCC. SDH-deficient RCC is negative for CK7, and loss of SDHB protein as determined by IHC (25). TFEB translocation RCC (26) is positive for cathepsin K, HMB45, Melan A, and TFEB. Molecular genetic analyses may detect TFEB gene expression. A new oncocytic type of FH-deficient RCC (27) exhibits variable cytoplasmic vacuolation, IHC shows strong nucleocytoplasmic 2SC positivity, PAX8 positivity and loss of FH expression, and can identify FH gene mutations. The broader differential types include epithelioid angiomyolipoma, CCRCC and PRCC with eosinophilic features, which are usually easily distinguished from LOT.
In summary, we present a 5-case series of LOT, an emerging entity of renal tumour with indolent clinical behaviour, which often presents a diagnostic challenge because renal tumours with oncocytic cytoplasm have a wide morphologic spectrum. The remarkable morphological features, immunohistochemical profile positive for CK7 and GATA3, and absent (or rarely weak) expression of CD117 and FOXI1 may help to avoid misdiagnosis. In challenging cases, the molecular genetic features of TSC/mTOR pathway mutations help to make the correct diagnosis.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TC: Design of the study and writing-original draft; TL and CW: Data acquisition and investigation; HW and YS: Data analysis and methodology; YP: Design of the study, writing-editing and reviewing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2023.1610852/full#supplementary-material
References
1.
Trpkov K Hes O . New and emerging renal entities: A perspective post-WHO 2016 classification. Histopathology (2019) 74(1):31–59. 10.1111/his.13727
2.
Trpkov K Williamson SR Gao Y Martinek P Cheng L Sangoi AR et al Low-grade oncocytic tumour of kidney (CD117-negative, cytokeratin 7-positive): A distinct entity? Histopathology (2019) 75(2):174–84. 10.1111/his.13865
3.
Akgul M Al-Obaidy KI Cheng L Idrees MT . Low-grade oncocytic tumour expands the spectrum of renal oncocytic tumours and deserves separate classification: A review of 23 cases from a single tertiary institute. J Clin Pathol (2021) 75:772–5. 10.1136/jclinpath-2021-207478
4.
Guo Q Liu N Wang F Guo Y Yang B Cao Z et al Characterization of a distinct low-grade oncocytic renal tumor (CD117-negative and cytokeratin 7-positive) based on a tertiary oncology center experience: The new evidence from China. Virchows Arch (2021) 478(3):449–58. 10.1007/s00428-020-02927-0
5.
Kravtsov O Gupta S Cheville JC Sukov WR Rowsey R Herrera-Hernandez LP et al Low-Grade Oncocytic Tumor of Kidney (CK7-Positive, CD117-Negative): Incidence in a single institutional experience with clinicopathological and molecular characteristics. Hum Pathol (2021) 114:9–18. 10.1016/j.humpath.2021.04.013
6.
Lerma LA Schade GR Tretiakova MS . Co-existence of ESC-RCC, EVT, and LOT as synchronous and metachronous tumors in six patients with multifocal neoplasia but without clinical features of tuberous sclerosis complex. Hum Pathol (2021) 116:1–11. 10.1016/j.humpath.2021.06.002
7.
Bai YF Chang CD Wang B Zhao M Teng XD . CK7+/CD117-low grade oncocytic tumor of the kidney: A clinicopathological analysis. Zhonghua Bing Li Xue Za Zhi (2022) 51(10):976–80. 10.3760/cma.j.cn112151-20220719-00627
8.
Kapur P Gao M Zhong H Chintalapati S Mitui M Barnes SD et al Germline and sporadic mTOR pathway mutations in low-grade oncocytic tumor of the kidney. Mod Pathol (2022) 35(3):333–43. 10.1038/s41379-021-00896-6
9.
Morini A Drossart T Timsit MO Sibony M Vasiliu V Gimenez-Roqueplo AP et al Low-grade oncocytic renal tumor (LOT): Mutations in mTOR pathway genes and low expression of FOXI1. Mod Pathol (2022) 35(3):352–60. 10.1038/s41379-021-00906-7
10.
Xie B Cheng LC Yin GL Liu BA Hu ZL Tong K . Clinicopathological features of low-grade oncocytic renal tumor (CD117-negative, cytokeratin 7-positive): Report of seven cases. Zhonghua Bing Li Xue Za Zhi (2022) 51(8):719–25. 10.3760/cma.j.cn112151-20220410-00263
11.
Zhang HZ Xia QY Wang SY Shi MJ . Low-grade oncocytic tumor of kidney harboring TSC/MTOR mutation: Clinicopathologic, immunohistochemical and molecular characteristics support a distinct entity. Virchows Arch (2022) 480(5):999–1008. 10.1007/s00428-022-03283-x
12.
Williamson SR Hes O Trpkov K Aggarwal A Satapathy A Mishra S et al Low-grade oncocytic tumour of the kidney is characterised by genetic alterations of TSC1, TSC2, MTOR or PIK3CA and consistent GATA3 positivity. Histopathology (2022) 82:296–304. 10.1111/his.14816
13.
Xia QY Wang XT Zhao M He HY Fang R Ye SB et al TSC/MTOR -associated eosinophilic renal tumors exhibit a heterogeneous clinicopathologic spectrum: A targeted next-generation sequencing and gene expression profiling study. Am J Surg Pathol (2022) 46(11):1562–76. 10.1097/PAS.0000000000001955
14.
WHO Classification of Tumours. Urinary and male genital tumors. 5th ed.Lyon (France): International Agency for Research on Cancer (2022).
15.
Tong K Hu Z . FOXI1 expression in chromophobe renal cell carcinoma and renal oncocytoma: A study of the cancer genome atlas transcriptome-based outlier mining and immunohistochemistry. Virchows Arch (2021) 478(4):647–58. 10.1007/s00428-020-02900-x
16.
Miettinen M McCue PA Sarlomo-Rikala M Rys J Czapiewski P Wazny K et al GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol (2014) 38(1):13–22. 10.1097/PAS.0b013e3182a0218f
17.
Mantilla JG Antic T Tretiakova M . GATA3 as a valuable marker to distinguish clear cell papillary renal cell carcinomas from morphologic mimics. Hum Pathol (2017) 66:152–8. 10.1016/j.humpath.2017.06.016
18.
Al-Obaidy KI Eble JN Cheng L Williamson SR Sakr WA Gupta N et al Papillary renal neoplasm with reverse polarity: A morphologic, immunohistochemical, and molecular study. Am J Surg Pathol (2019) 43(8):1099–111. 10.1097/PAS.0000000000001288
19.
Mansoor M Siadat F Trpkov K . Low-grade oncocytic tumor (LOT) - a new renal entity ready for a prime time: An updated review. Histol Histopathol (2022) 37(5):405–13. 10.14670/HH-18-435
20.
Miricescu D Balan DG Tulin A Stiru O Vacaroiu IA Mihai DA et al PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (Review). Exp Ther Med (2021) 21(5):540. 10.3892/etm.2021.9972
21.
Pivovarcikova K Alaghehbandan R Vanecek T Ohashi R Pitra T Hes O . TSC/mTOR pathway mutation associated eosinophilic/oncocytic renal neoplasms: A heterogeneous group of tumors with distinct morphology, immunohistochemical profile, and similar genetic background. Biomedicines (2022) 10(2):322. 10.3390/biomedicines10020322
22.
Trpkov K Abou-Ouf H Hes O Lopez JI Nesi G Comperat E et al Eosinophilic solid and cystic renal cell carcinoma (ESC RCC): Further morphologic and molecular characterization of ESC RCC as a distinct entity. Am J Surg Pathol (2017) 41(10):1299–308. 10.1097/PAS.0000000000000838
23.
He H Trpkov K Martinek P Isikci OT Maggi-Galuzzi C Alaghehbandan R et al High-grade oncocytic renal tumor": Morphologic, immunohistochemical, and molecular genetic study of 14 cases. Virchows Arch (2018) 473(6):725–38. 10.1007/s00428-018-2456-4
24.
Farcas M Gatalica Z Trpkov K Swensen J Zhou M Alaghehbandan R et al Eosinophilic vacuolated tumor (EVT) of kidney demonstrates sporadic TSC/MTOR mutations: Next-generation sequencing multi-institutional study of 19 cases. Mod Pathol (2022) 35(3):344–51. 10.1038/s41379-021-00923-6
25.
Tsai TH Lee WY . Succinate dehydrogenase-deficient renal cell carcinoma. Arch Pathol Lab Med (2019) 143(5):643–7. 10.5858/arpa.2018-0024-RS
26.
Xia QY Wang XT Fang R Wang Z Zhao M Chen H et al Clinicopathologic and molecular analysis of the TFEB fusion variant reveals new members of TFEB translocation renal cell carcinomas (RCCs): Expanding the genomic spectrum. Am J Surg Pathol (2020) 44(4):477–89. 10.1097/PAS.0000000000001408
27.
Smith SC Sirohi D Ohe C McHugh JB Hornick JL Kalariya J et al A distinctive, low-grade oncocytic fumarate hydratase-deficient renal cell carcinoma, morphologically reminiscent of succinate dehydrogenase-deficient renal cell carcinoma. Histopathology (2017) 71(1):42–52. 10.1111/his.13183
Summary
Keywords
immunohistochemistry, clinicopathological characteristics, mTOR, differential diagnosis, low-grade oncocytic tumour (LOT) of the kidney
Citation
Chen T, Peng Y, Lei T, Wu C, Wang H and Shi Y (2023) Low-grade oncocytic tumour (LOT) of the kidney is characterised by GATA3 positivity, FOXI1 negativity and mTOR pathway mutations. Pathol. Oncol. Res. 29:1610852. doi: 10.3389/pore.2023.1610852
Received
29 September 2022
Accepted
12 January 2023
Published
01 February 2023
Volume
29 - 2023
Edited by
Gabor Cserni, University of Szeged, Hungary
Updates
Copyright
© 2023 Chen, Peng, Lei, Wu, Wang and Shi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Peng, pengyan871005@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.