Abstract
Introduction: High-risk patients with grade 3 endometrioid endometrial carcinoma (G3EEC) who require adjuvant therapy have not been clearly identified. Therefore, the current study aimed to investigate the prognostic impact of ARID1A, p53, and mismatch repair (MMR) protein expressions, previously reported as prognosticators in some gynecological cancers, in patients with early-stage G3EEC.
Methods: A total of 67 patients with pathologically confirmed early-stage G3EEC diagnosed between 1997 and 2020 were identified; none received adjuvant chemotherapy. The recurrence-free survival (RFS) and overall survival (OS) were estimated using the Kaplan-Meier method and compared with a log-rank test. The protein expressions of ARID1A, p53, and MMR were examined via immunohistochemistry, and the associations between these biomarkers and clinical outcomes were evaluated.
Results: Recurrence was observed in 9 (13%) of the 67 patients with early stage G3EEC. The respective 5-years RFS and OS rates were 87.7% and 93.7%, and 68.6% and 85.7%, respectively for stages I and II. Multivariate analysis showed significantly longer RFS among patients with ARID1A loss (hazard ratio = 8.7; 95% CI, 1.09–69.6, p = 0.04). No significant differences were observed in RFS and OS of patients according to p53 and MMR expression status.
Conclusion: ARID1A expression status was a prognosticator for patients with early stage G3EEC without adjuvant therapy, whereas p53 and MMR expression status showed no impact on survival outcomes. ARID1A may become a useful biomarker for stratification of adjuvant treatment for early stage G3EEC patients.
Introduction
The prevalence of endometrial cancer has been increasing worldwide, with over 380,000 new cases diagnosed in 2018 [1]. In Japan, endometrial cancer is the most commonly observed gynecologic malignancy [2]. Endometrial cancers have been classified as type 1 or type 2 on the basis of the associated risk factors and prognosis [2]. In contrast to type 1, type 2 endometrial cancer is typically not estrogen-driven, and clinically behaves more aggressively.
Grade 3 endometrioid endometrial carcinoma (G3EEC) is conventionally categorized as a type 2 endometrial cancer along with uterine serous carcinoma (USC) and clear cell carcinoma (CCC). Some case series reported similar survival outcomes between G3EEC, USC, and CCC [3–8]. However, in other studies, particularly a retrospective review that used data obtained from the Surveillance, Epidemiology, and End Results Program, patients with G3EEC had a significantly better overall survival (OS) rate than that of patients with USC or CCC [9–11]. Owing to these conflicting results, adjuvant therapy implementation for early-stage G3EEC is controversial; therefore, it is important to clarify the prognostic factors of early stage G3EEC.
Negative prognostic factors of endometrial carcinoma include non-estrogen dependent tumors (type 2 endometrial cancer), elderly age, and advanced International Federation of Gynecology and Obstetrics (FIGO) stage [12]. Additionally, the expression status of ARID1A, p53, and mismatch repair (MMR) proteins can be independent prognosticators [13, 14].
Significant differences were observed between FIGO stages I-IV clear cell and endometrioid subtypes of ovarian and endometrial cancer according to the protein expression status of ARID1A. Several studies reported that ARID1A mutations correlated with a favorable survival outcome [15, 16]. In addition, aberrant p53 expression was significantly associated with a higher tumor grade and shorter OS [13]. Moreover, MMR deficiency (dMMR) was associated with elderly age, higher tumor grade (G3), and advanced stage (II-IV), whereas MLH1 promoter hypermethylation predicted a shorter disease-specific survival [14].
However, these studies included all histologic types and stages of endometrial cancer. Only a few studies evaluated the association between prognosis and expression of ARID1A, p53, and MMR, specifically for early-stage G3EEC patients.
In the current study, we retrospectively reviewed patients with early-stage G3EEC and performed immunohistochemistry (IHC) for ARID1A, p53, and MMR proteins. Notably, we could evaluate the baseline recurrence risk in our patients because none of them received adjuvant treatment during the study period due to the lack of compelling evidence in its favor. The study aimed to clarify the prognostic impact of the expression status of ARID1A, p53, and MMR proteins on the outcomes of patients with early-stage G3EEC.
Methods
Patient Demographic Characteristics
The study was approved by the Institutional Review Board of the National Cancer Center in Japan (#2016-260) and conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature.
Patients with pathologically confirmed stage I and stage II (per the 2008 FIGO classification) G3EEC diagnosed between 1997 and 2020 were identified from the tumor registry database of our institution. All tumors were surgically resected by gynecologic oncologists. All surgical staging procedures, including total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node sampling, were performed via laparotomy. At least two gynecologic pathologists confirmed the histopathological diagnosis of G3EEC and absence of lymph node metastases. Although the Japanese treatment guidelines for endometrial cancer recommend adjuvant chemotherapy for stage IA and IB/II G3EEC, there is no strong evidence supporting this treatment strategy [17]. Thus, the patients included in the present study did not receive adjuvant treatment. The clinicopathological data were collected via a retrospective review of medical charts.
The follow-up for most patients comprised vaginal inspections, Pap smear cytology, and radiological examination including chest radiography every 3-6 months, and computed tomography (CT) of the chest, abdomen, and pelvis every 6 months for the first 2 years. During the third and fourth years of follow-up, patients underwent vaginal examinations, two Pap smears, and annual radiological examinations. After five years of follow-up, patients underwent vaginal inspections and Pap smears annually, and those with suspected G3EEC recurrence underwent CT of the chest to pelvis, magnetic resonance imaging, and histological examination.
Immunohistochemistry Analysis and Interpretation
All the surgically resected specimens were fixed in 10% neutral-buffered formalin for 24–72 h and embedded in paraffin. One representative whole 4 μm-thick section was analyzed using IHC. The following antibodies were used for IHC on the representative slides for each case: anti-p53 (DO7, pre-diluted; Dako, Glostrup, Denmark); anti-ARID1A (polyclonal, 1:2,000 dilution; Sigma, St. Louis, MO, United States); anti-hMSH6 (SP93, 1:200 dilution; Spring Bioscience, CA, United States); and anti-hPMS2 (A16-4, 1:200 dilution; Biocare Medical, CA, United States) antibodies. We performed all the IHC tests using a Dako autostainer (Dako, CA, United States) according to the manufacturer’s recommendations. After deparaffinization, tissue sections were stained using the antibodies described above, and then counterstained with hematoxylin.
Aberrant p53 staining pattern was defined as a strong and diffuse nuclear staining pattern (>70% of carcinoma cells) or completely negative (“null pattern”) staining of carcinoma cells with appropriate staining of surrounding non-tumor cells as an internal positive control. A weak and heterogeneous staining pattern of tumor cells was classified as the wild-type pattern.
We assessed ARID1A expression by immunohistochemistry and categorized the results into 3 classes, namely, retained expression (positivity in almost all tumor cells), homogenous loss of expression (negativity in almost all or >90% of tumor cells), and heterogeneous loss of expression (regional negativity with 10–90% of ARID1A-negative tumor cells). Both, homogenous and heterogenous loss of ARID1A expression were regarded as ARID1A loss.
IHC for PMS2 and MSH6 alone can reportedly replace a four antibody panel (comprising MLH1, MSH2, MSH6, and PMS2) for dMMR screening [18]; therefore, in the present study, MMR-deficient status was defined as the complete loss of nuclear staining for PMS2 and/or MSH6 proteins. Adjacent normal mucosa, stromal cells, and inflammatory cells with intact nuclear staining served as internal positive controls. Representative images of IHC for p53, ARID1A, and MMR proteins are depicted in Figure 1.
FIGURE 1
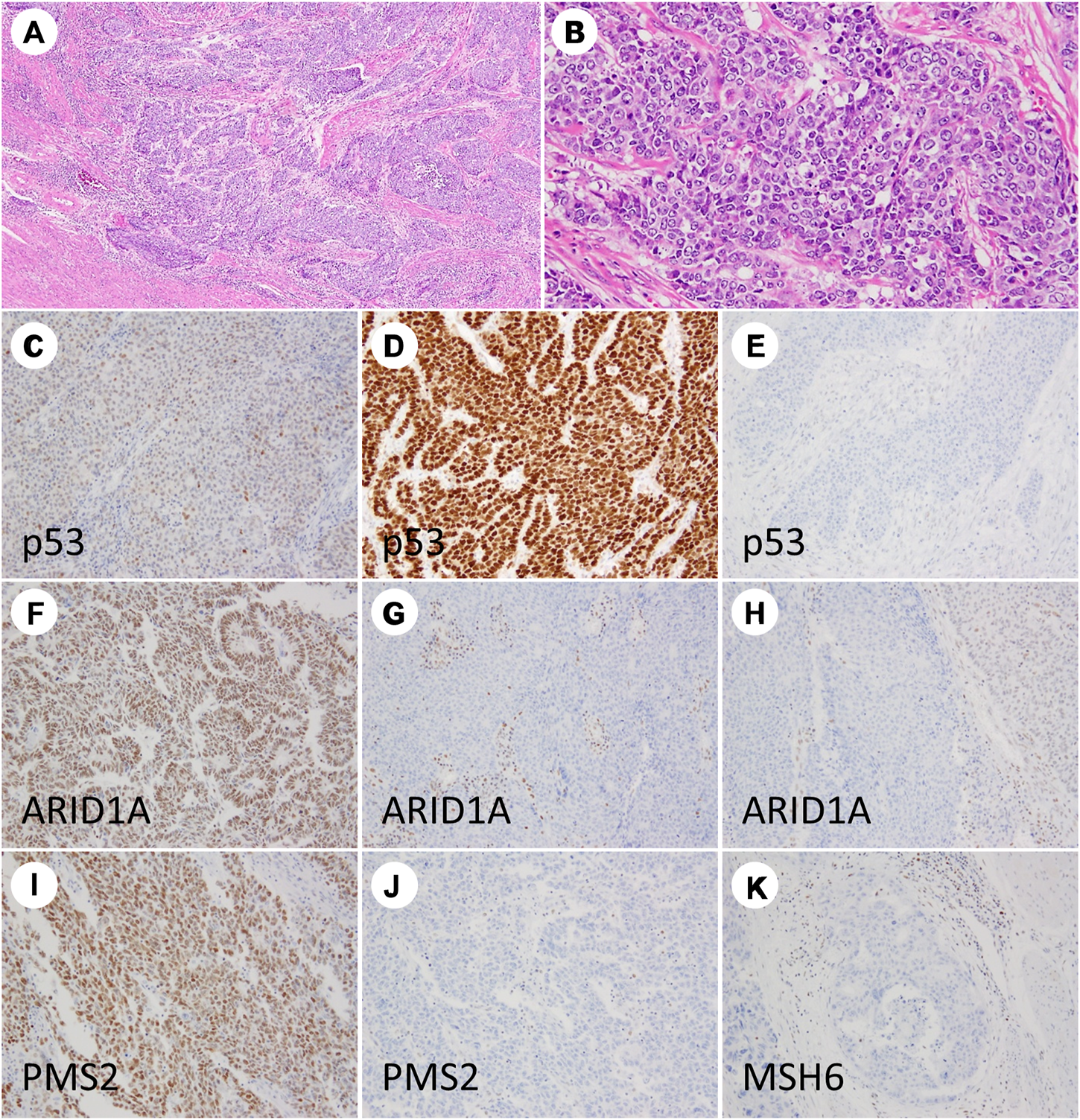
Immunohistochemistry staining results of grade 3 endometrioid endometrial carcinoma. The tumor is dominantly composed of solid nests (A,B). TP53 showing wild-type (C), diffuse strong (D), and null patterns (E). ARID1A showing intact staining (F), complete loss (G), and partial loss (H). PMS2 nuclear staining is retained (I) and lost (J). MSH6 staining is lost (K).
Statistical Analysis
The follow-up time and time to events were measured from the date of surgery to the last known visit, confirmation of recurrence, or death. Recurrence-free survival (RFS) was defined as the time from the date of surgery to the date of the first recurrence. OS was defined as the time from the date of surgery to the date of death due to any cause. Survival values were estimated using the Kaplan-Meier method and compared with log-rank tests. Independent prognostic factors for RFS were determined using Cox proportional hazards regression models. All analyses were performed using the JMP14 software program (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Patient Demographic Characteristics
Between 1997 and 2020, a total of 113 patients were diagnosed with G3EEC. All surgical specimens were histologically reviewed, and immunostaining was performed for 110 patients with available specimens.
Among the 113 patients with G3EEC, 67 were identified with FIGO stage I and II disease. None of the patients with early-stage disease received adjuvant treatment; the median age was 58 years (range, 43–80 years). The clinicopathological features of the patients with stage I–II disease are summarized in Table 1.
TABLE 1
| Characteristic | No (%) (n = 67) | |
|---|---|---|
| Stage (FIGO 2008) | IA | 35 (52) |
| IB | 25 (37) | |
| II | 7 (10) | |
| Age, years | <65 | 47 (70) |
| ≥65 | 20 (30) | |
| BMI | <30 | 62 (93) |
| ≥30 | 5 (7) | |
| Depth of invasion | Endometrium | 5 (7) |
| Myometrium <50% | 31 (46) | |
| Myometrium ≥50% | 31 (46) | |
| Cervical stromal involvement | Present | 7 (10) |
| Absent | 60 (90) | |
| LVSI | Present | 42 (63) |
| Absent | 25 (37) | |
| Pelvic cytology | Positive | 8 (12) |
| Negative | 59 (88) | |
Characteristics of patients with early stage grade 3 endometrioid endometrial carcinoma.
BMI, body mass index; FIGO, international federation of gynecology and obstetrics; LVSI, lymphovascular space invasion
Treatment and Prognosis of Patients With Early-Stage G3EEC
All patients with early-stage G3EEC underwent total hysterectomy and bilateral salpingo-oophorectomy. Pelvic lymph node sampling was performed in 60 patients (89.6%). Seven patients did not undergo lymph node sampling due to their advanced age and complications. The median number of removed lymph nodes was 29 (range, 1–58). The median follow-up time for patients with early-stage G3EEC was 65 months (range, 3–182 months). Recurrence was observed in nine patients (13.4%), and the median time to recurrence was 22 months (range, 9–45 months). Recurrences in the vagina, lymph nodes, and other distant sites were observed in one, three, and five patients, respectively.
The clinicopathological features, treatment, and prognosis of the patients with recurrence are summarized in Table 2. The five-year RFS rates for patients with stage I and II disease were 87.7 and 68.6%, respectively. The five-year OS rates for patients with stage I and II disease were 93.7 and 85.7%, respectively. The median RFS and OS were 63.2 months (range, 3–220 months) and 65.6 months (range, 3–227 months), respectively.
TABLE 2
| Case | Age | Stage | Tumor size (mm) | Depth of invasion | LVSI | p53 IHC | ARID1A IHC | MMR status | Site of recurrence | Treatment for recurrence | DFS (mo) | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | IA | 33 | <1/2 | Present | Wild type | Lost (homo) | deficient (PMS2) | Bone | RT + Chemotherapy | 12 | LOF, 17 months |
| 2 | 57 | II | 35 | ≥1/2 | Absent | Wild type | Retained | Proficient | lung (44mo), abdominal mass (96mo) | Surgery + Chemotherapy | 44 | NED, 182 months |
| 3 | 65 | IB | 120 | ≥1/2 | Present | Wild type | Retained | Proficient | PAN | Surgery + Chemotherapy | 13 | NED, 69 months |
| 4 | 70 | II | 66 | ≥1/2 | Present | Diffuse | Retained | Proficient | PLN | MPA | 17 | DOD, 32 months |
| 5 | 67 | IB | 40 | ≥1/2 | Present | Wild type | Retained | Proficient | peritoneum + pleura | Chemotherapy | 21 | DOD, 40 months |
| 6 | 69 | IB | 15 | ≥1/2 | Present | Wild type | Retained | Proficient | Lung | Chemotherapy | 27 | DOD, 47 months |
| 7 | 54 | IB | 70 | ≥1/2 | Present | Wild type | Retained | Deficient (PMS2) | Vagina | RT | 6 | NED, 45 months |
| 8 | 54 | IB | 70 | ≥1/2 | Present | Diffuse | Retained | Proficient | Liver, PAN | Chemotherapy | 10 | NED, 23 months |
| 9 | 71 | IB | 40 | ≥1/2 | Present | Diffuse | Retained | Proficient | Lung | Chemotherapy | 9 | AWD, 9 months |
Clinicopathological characteristics, treatment, and outcomes of patients with recurrence of early-stage grade 3 endometrioid endometrial carcinoma.
LVSI, lymphovascular space invasion; ARID1A, AT-rich interaction domain 1A; MMR, mismatch repair; DFS, disease-free survival; LOF, lost to follow-up; NED, no evidence of disease; PAN, para-aortic lymph node; PLN, pelvic lymph node; MPA, medroxyprogesterone acetate; DOD, died of disease
Immunohistochemistry for ARID1A, p53, and MMR Proteins
The results of IHC staining of early stage G3EEC are shown in Table 3. An aberrant expression pattern of p53 (overexpression or complete absence) was observed in 21 of 67 cases (31.3%), loss of MMR protein expression was observed in 40.3% of cases, isolated PMS2 loss was observed in 22 of 27 dMMR cases (81.5%), and loss of ARID1A expression was observed in approximately half of the patients (50.7%). There were no significant correlations between ARID1A, p53, and MMR expression status. Moreover, the expression status of these biomarkers was not correlated with clinicopathological features such as tumor size (greatest dimension), depth of myometrial invasion, cervical stromal invasion, lymphovascular invasion, and intraoperative peritoneal cytology.
TABLE 3
| Marker | Staining pattern | No (%) (n = 67) |
|---|---|---|
| p53 | Aberrant pattern | 21 (31) |
| Diffuse pattern | 20 (29) | |
| Null pattern | 1 (1) | |
| Wild-type pattern | 46 (69) | |
| ARID1A | Retained | 33 (49) |
| Lost | 34 (51) | |
| Homogenous pattern | 22 | |
| Heterogenous pattern | 12 | |
| MMR | Proficient | 40 (60) |
| Deficient | 27 (40) | |
| PMS2 | 22 | |
| MSH6 | 5 |
Number of patients showing immunohistochemical staining of early-stage grade 3 endometrioid endometrial carcinoma.
ARID1A: AT-rich interaction domain 1A, MMR: mismatch repair.
Association Between Prognosis and The ARID1A, p53 and MMR Expression Status
Kaplan-Meier analysis showed retained ARID1A expression predicting lower RFS in the whole cohort (75% vs. 97%, p = 0.04, Table 4; Figure 2). Multivariate Cox regression analysis identified ARID1A status as an independent prognostic factor with a hazard ratio of 8.7 (95% CI 1.09–69.6). No significant correlations were found between recurrence and p53 and MMR expression status (Table 4; Figures 3, 4). Furthermore, survival analysis revealed no significant differences among patients according to ARID1A, p53, and MMR expression status (Supplementary Table S1).
TABLE 4
| Variable | Category | N | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| (p value) | HR | 95% CI | p value | |||
| Age | <65 | 47 | 0.07 | |||
| ≥65 | 20 | |||||
| Stage | I | 60 | 0.82 | |||
| II | 7 | |||||
| Depth of invasion | <1/2 | 36 | 0.01 | 1 | ||
| ≥1/2 | 31 | 9.9 | 1.23–79.2 | 0.03 | ||
| LVSI | Negative | 25 | 0.1 | |||
| Positive | 42 | |||||
| p53 IHC | Diffuse/Null | 21 | 0.83 | |||
| Wild type | 46 | |||||
| ARID1A IHC | Lost | 34 | 0.01 | 1 | ||
| Retained | 33 | 8.7 | 1.09–69.6 | 0.04 | ||
| MMR IHC | Proficient | 40 | 0.28 | |||
| Deficient | 27 | |||||
Univariate and multivariate analyses of factors suspected to affect recurrence-free survival.
LVI, lymphovascular space invasion; IHC, immunohistochemistry; ARID1A, AT-rich interaction domain 1A; MMR, mismatch repair.
FIGURE 2
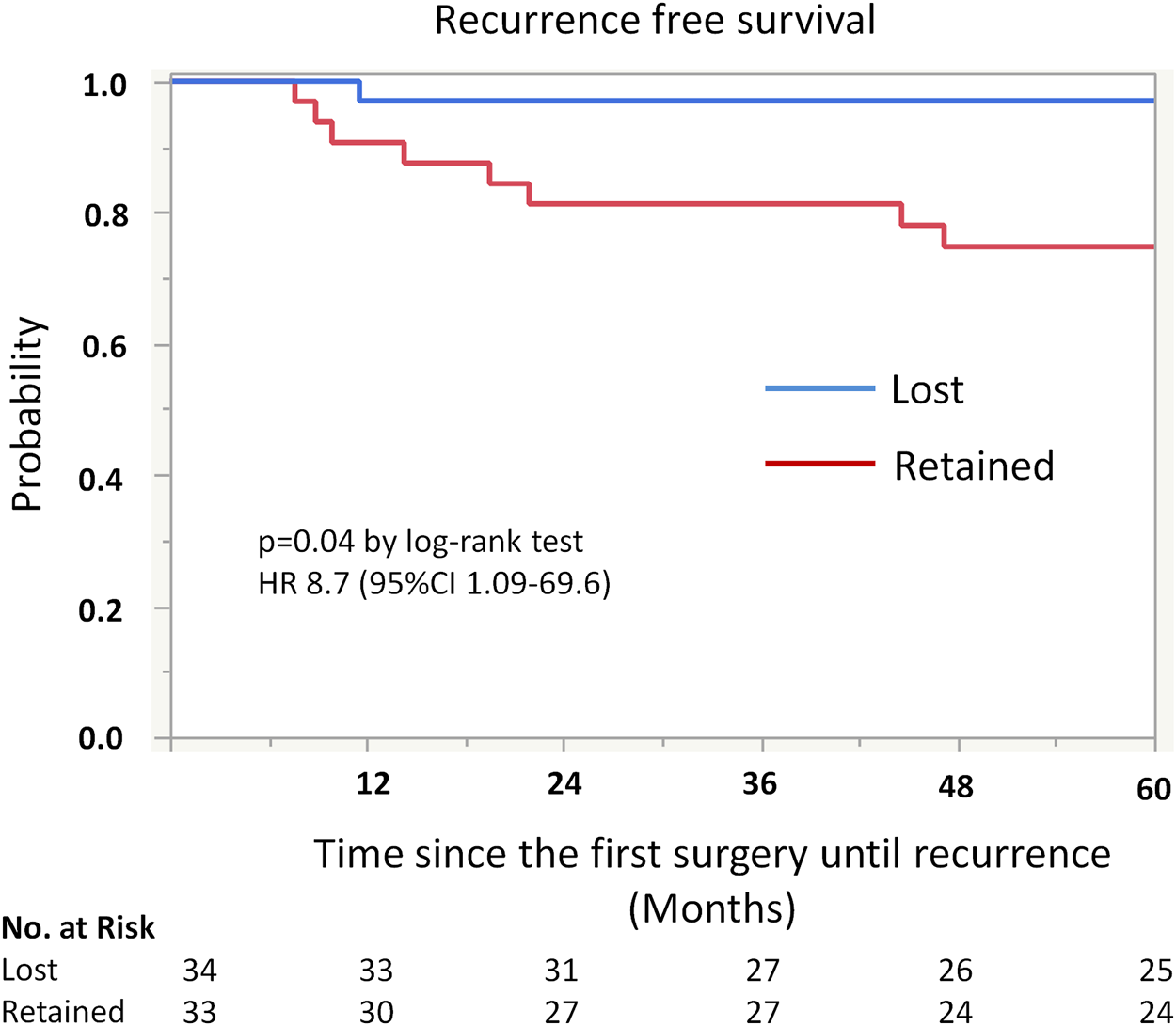
Kaplan-Meier curve for recurrence free survival (RFS) by ARID1A status. The 5-year recurrence-free survival for patients with retained ARID1A expression was 75%. On the other hand, the 5-year recurrence-free survival for those who were ARID1A lose was 97% (p = 0.04).
FIGURE 3
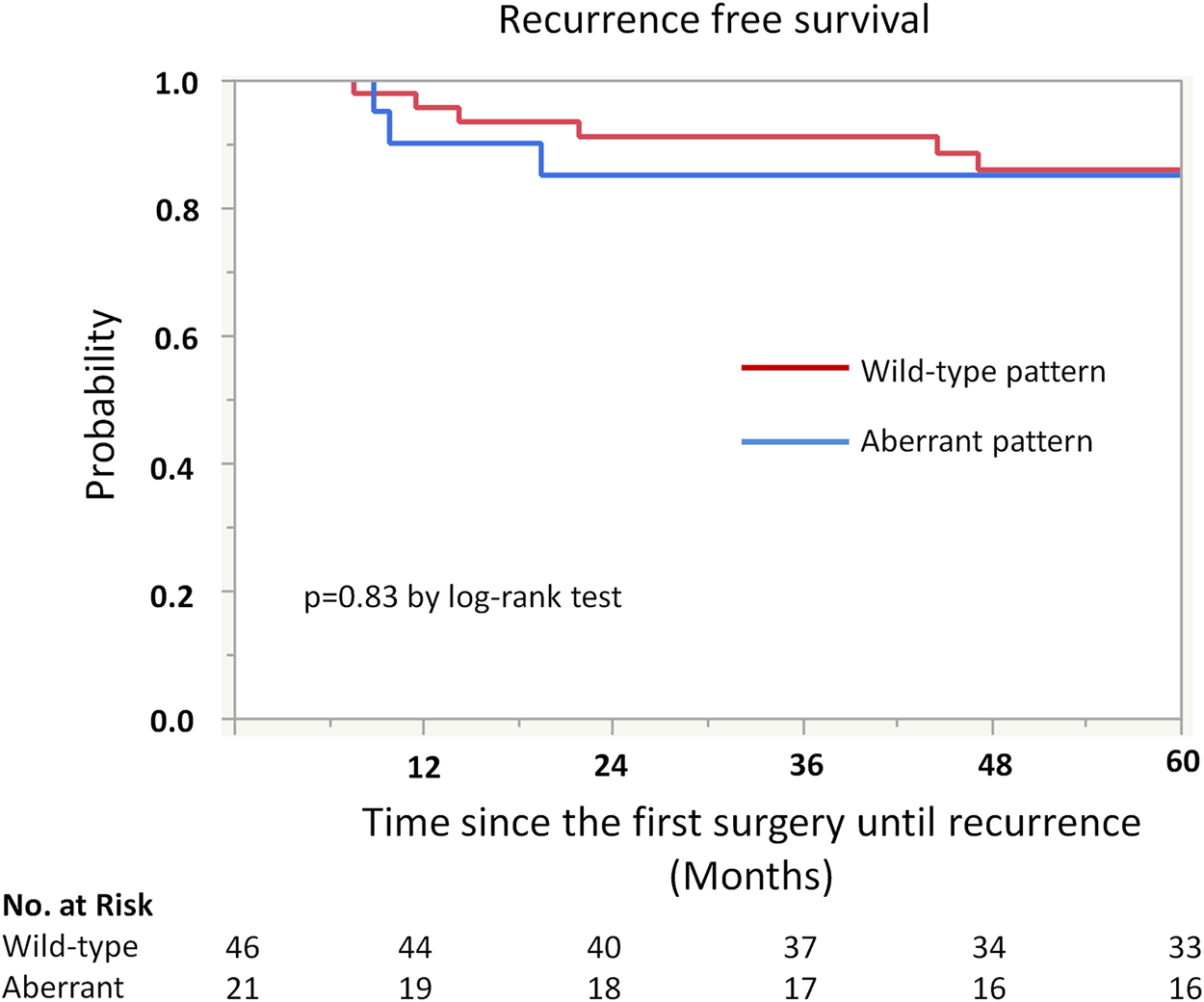
Kaplan-Meier curve for recurrence free survival (RFS) by p53 status.
FIGURE 4
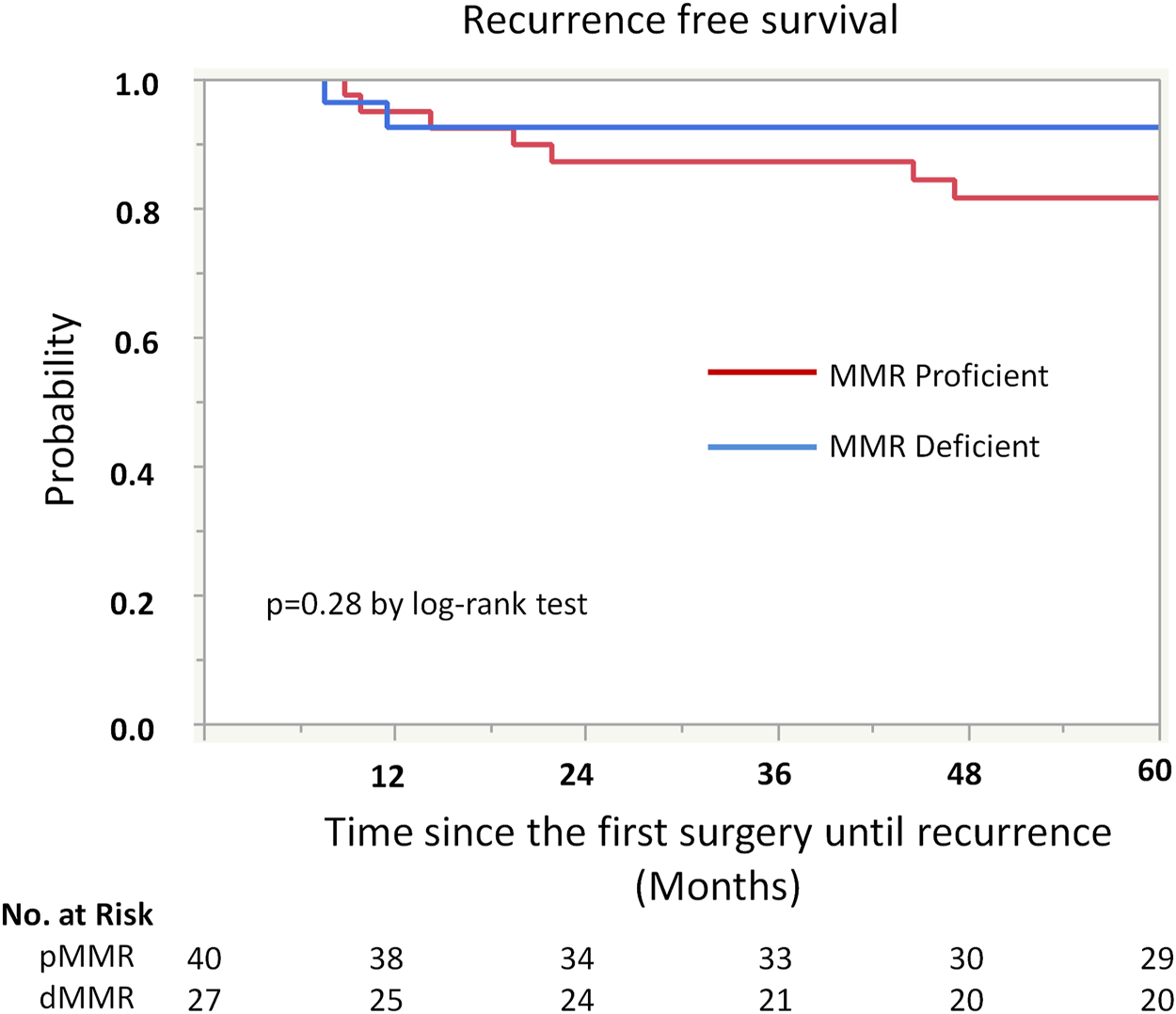
Kaplan-Meier curve for recurrence free survival (RFS) by mismatch repair (MMR) protein expression status.
Discussion
In the present study, early-stage G3EEC patients had a favorable prognosis. The five-year RFS rates for patients with stage I and II disease were 87.7 and 68.6%, respectively, while the corresponding 5-year OS rates were 93.7 and 85.7%. Loss of ARID1A expression was associated with significantly longer RFS, whereas p53 and MMR proteins expression showed no impact on the five-year RFS and OS rates.
The survival rates for early-stage patients who did not receive any adjuvant therapy were comparable to or better than those reported in previous studies, including patients who received adjuvant therapy (Supplementary Table S2) [2, 4–6, 9, 19]. As the pathological diagnosis of G3EEC is difficult and necessitates interobserver variability [20], a central pathology review is recommended in studies, including cases of high-grade endometrial carcinoma such as G3EEC. In our study, the higher rate of lymph node sampling and detection procedures (90%) yielded more favorable outcomes than previous studies [2, 4].
This study revealed the significant prognostic capabilities of ARID1A expression in early stage G3EEC cases. ARID1A is a recently identified tumor suppressor gene on chromosome 1q36, which participates in forming SWI/SNF chromatin complexes and regulating gene expression. The inactivation of ARID1A promotes the cell cycle and contributes to carcinogenesis [21]. ARID1A mutations were reported in 30–50% of low-grade EECs and 40–60% of high-grade EECs [22, 23]. Furthermore, the loss of ARID1A protein was reported in 29% of low-grade EECs and 39% of high-grade EECs [24]. Mao et al. revealed that ARID1A expression was retained in benign endometrial tissues, but lost in endometrioid intraepithelial neoplasia, low-grade EECs, and high-grade EECs [25]. These observations suggest that ARID1A loss predisposes endometrial carcinogenesis. Moreover, Mao et al. reported a significant increase in the complete loss of ARID1A in the high-grade component of high-grade EECs accompanied with low-grade carcinoma components comprising retained or clonal loss of ARID1A. It indicates that ARID1A loss plays a role in tumor progression [25]. Collectively, ARID1A loss reflected biological aggressiveness of gynecological cancers. However, the prognostic capability of ARID1A loss has not been documented in endometrial cancer.
The prognostic impact of ARID1A loss in cohorts of patients with endometrial cancer of various histological types and/or stages was rarely observed. From a cohort of 535 primary and 77 metastatic endometrial cancer patients, Werner et al. [26] reported significant correlations between ARID1A loss and younger patient age and deeper myometrial invasion, but not survival. In contrast, Zhang et al. [16] observed no associations between ARID1A loss and clinical stage, depth of myometrial invasion, lymph node metastasis, or OS among endometrial carcinoma patients. Notably, Shen et al. [15] demonstrated that ARID1A mutations correlate with favorable survival in endometrial carcinoma using data from The Cancer Genome Atlas. Additionally, they associated ARID1A mutations with better prognosis in patients with microsatellite stable tumors [15]. Interestingly, seven of nine cases with recurrence in our cohort also showed retained ARID1A expression and pMMR phenotype. In addition to MMR deficiency, DNA Polymerase Epsilon and Catalytic Subunit POLE mutation is another favorable prognostic factor, that can be considered in relation to ARID1A loss. Although our study did not include data on mutations, the POLE mutation has been reportedly observed in approximately 10% of endometrial cancers with ARID1A alteration [27]. Therefore, a minor subgroup of cases showing ARID1A loss may have a favorable prognosis attributable to POLE mutations. However, most cases with ARID1A loss do not seem to have a co-existing POLE mutation. Recently, POLE has been reported as one of the putative downstream effectors of ARID1A [28]. In line with this, ARID1A-mutant tumors have been consistently reported as having higher mutation load than that of ARID1A-intact tumors [15, 27]. Collectively, ARID1A loss reportedly promotes mutagenicity of the tumor and may induce a similar phenotype to that of POLE-mutated or MMR-deficient tumors, that may play a key role in endometrial carcinogenesis. Interestingly, co-occurring landscapes of genetic mutations reported in ARID1A-mutated cancers were completely different in early-stage versus advanced-stage cancer [27]. Molecular alterations accompanied with ARID1A loss in each stage of endometrial cancer should be elucidated in future investigations to clarify the role of ARID1A in endometrial carcinogenesis and tumor progression.
Significant prognostic impact of p53 expression status was not observed. An abnormal p53 pattern on IHC staining is an independent poor prognostic factor among endometrial and ovarian cancer patients [29]. Moreover, mutational analysis of G3EEC identified TP53 as an independent prognostic factor for poor RFS [30]. However, this study included patients treated with different types of adjuvant therapy and did not stratify survival analysis by adjuvant treatment. Consequently, its findings cannot be compared with those of the present study. In our study, early-stage G3EEC patients had a favorable prognosis regardless of p53 expression status. According to The Cancer Genome Atlas data on endometrioid endometrial carcinoma, approximately 8% of cases may have POLE mutations, partially including concurrent p53 mutation, and show a favorable prognosis [22]. Since POLE mutation analysis was not performed in the present study, a few cases with concurrent p53 and POLE mutations may compromise the adverse prognostic impact of abnormal p53 expression status. Nevertheless, our finding suggests that IHC analysis of p53 alone was not useful for risk stratification of early-stage G3EEC patients after surgery.
No significant prognostic impact of MMR protein expression was found. Abnormalities in the DNA MMR gene is a hallmark of molecular pathway to carcinogenesis, being observed in 20–40% of cases of endometrial carcinoma [31]. Germline mutations (Lynch syndrome) comprise 3–5% of all defective DNA MMR. The remaining cases are caused by promoter hypermethylation of MLH1, leading to instability of microsatellites [14]. Results associating MMR loss of function with prognosis differ across previous studies [32–36]. While the presence of microsatellite instability was independently associated with a more favorable clinical outcome in endometrial carcinoma [35], other studies demonstrated that young women (younger than 40 or 60 years) with loss of MMR proteins were at risk of high-grade tumors with poor clinical outcomes [32–34]. Moreover, a meta-analysis, including all FIGO tumor stages showed no definitive evidence for a significant association between MMR status and survival outcomes in patients with endometrial cancer [37]. These discrepancies may be attributed to the heterogeneity of the study population. Notably, the current study only included early stage G3EEC patients, who did not receive adjuvant therapy; no significant association was observed between MMR status and prognosis in this population.
Limitations of this study include its single-center retrospective design and the low statistical power attributed to the small number of events. Despite these, this study provided meaningful data regarding the natural clinical course and baseline recurrence risk of early stage G3EEC, by including the largest cohort of pathologically confirmed early-stage G3EEC patients who did not receive adjuvant therapy.
In conclusion, the loss of ARID1A expression favorably prognosticated early-stage G3EEC patients who did not undergo adjuvant therapy. In contrast, p53 and MMR expressions did not show significant prognostic impact on the five-year RFS and OS rates. Although the molecular basis of the relation between ARID1A loss and favorable prognosis remains unclear, the ARID1A expression status may become a useful marker for stratification of adjuvant treatment in early-stage G3EEC patients.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the National Cancer Center, Tokyo, Japan (#2016-260). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MK and HY. The first draft of the manuscript was written by MK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP20K18207 and JP19K16572.
Acknowledgments
We would like to thank Sachiko Miura, MT, Toshiko Sakaguchi, MT, and Chizu Kina, MT for technical assistance. We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2021.598550/full#supplementary-material.
References
1.
Siegel RL Miller KD Jemal A . Cancer statistics, 2019. CA A Cancer J Clin (2019). 69(1):7–34. 10.3322/caac.21551
2.
McGunigal M Liu J Kalir T Chadha M Gupta V . Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: a national cancer database analysis. Int J Gynecol Cancer (2017). 27(1):85–92. 10.1097/IGC.0000000000000844
3.
Voss MA Ganesan R Ludeman L McCarthy K Gornall R Schaller G et al Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol (2012). 124(1):15–20. 10.1016/j.ygyno.2011.07.030
4.
Creasman WT Kohler MF Odicino F Maisonneuve P Boyle P . Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol (2004). 95(3):593–6. 10.1016/j.ygyno.2004.08.019
5.
Alektiar KM McKee A Lin O Venkatraman E Zelefsky MJ McKee B et al Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO Grade 3 cancer? Int J Radiat Oncology*Biology*Physics (2002). 54(1):79–85. 10.1016/s0360-3016(02)02913-9
6.
Cirisano FD Jr Robboy SJ Dodge RK Bentley RC Krigman HR Synan IS et al The outcome of stage I-II clinically and surgically staged papillary serous and clear cell endometrial cancers when compared with endometrioid carcinoma. Gynecol Oncol (2000). 77(1):55–65. 10.1006/gyno.2000.5737
7.
Soslow RA Bissonnette JP Wilton A Ferguson SE Alektiar KM Duska LR et al Clinicopathologic analysis of 187 high-grade endometrial carcinomas of different histologic subtypes: similar outcomes belie distinctive biologic differences. Am J Surg Pathol (2007). 31(7):979–87. 10.1097/PAS.0b013e31802ee494
8.
Alvarez T Miller E Duska L Oliva E . Molecular profile of grade 3 endometrioid endometrial carcinoma. Am J Surg Pathol (2012). 36(5):753–61. 10.1097/PAS.0b013e318247b7bb
9.
Hamilton CA Cheung MK Osann K Chen L Teng NN Longacre TA et al Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer (2006). 94(5):642–6. 10.1038/sj.bjc.6603012
10.
Alkushi A Köbel M Kalloger SE Gilks CB . High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol (2010). 29(4):343–50. 10.1097/PGP.0b013e3181cd6552
11.
Boruta DM 2nd Gehrig PA Groben PA Bae-Jump V Boggess JF Fowler WC Jr et al Uterine serous and grade 3 endometrioid carcinomas. Cancer (2004). 101(10):2214–21. 10.1002/cncr.20645
12.
Saso S Chatterjee J Georgiou E Ditri AM Smith JR Ghaem-Maghami S . Endometrial cancer. BMJ (2011). 343:d3954. 10.1136/bmj.d3954
13.
Heckl M Schmoeckel E Hertlein L Rottmann M Jeschke U Mayr D . The ARID1A, p53 and ss-Catenin statuses are strong prognosticators in clear cell and endometrioid carcinoma of the ovary and the endometrium. PLoS One (2018). 13(2):e0192881. 10.1371/journal.pone.0192881
14.
Pasanen A Loukovaara M Bützow R . Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod Pathol (2020). 33:1443. 10.1038/s41379-020-0501-8
15.
Shen J Ju Z Zhao W Wang L Peng Y Ge Z et al ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med (2018). 24(5):556–62. 10.1038/s41591-018-0012-z
16.
Zhang Z-M Xiao S Sun G-Y Liu Y-P Zhang F-P Yang H-F et al The clinicopathologic significance of the loss of BAF250a (ARID1A) expression in endometrial carcinoma. Int J Gynecol Cancer (2014). 24(3):534–40. 10.1097/IGC.0000000000000092
17.
Susumu N Sagae S Udagawa Y Niwa K Kuramoto H Satoh S et al Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol (2008). 108(1):226–33. 10.1016/j.ygyno.2007.09.029
18.
Hall G Clarkson A Shi A Langford E Leung H Eckstein RP et al Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma. Pathology (2010). 42(5):409–13. 10.3109/00313025.2010.493871
19.
Rasool N Fader AN Seamon L Neubauer NL Shahin FA Alexander HA et al Stage I, grade 3 endometrioid adenocarcinoma of the endometrium: an analysis of clinical outcomes and patterns of recurrence. Gynecol Oncol (2010). 116(1):10–4. 10.1016/j.ygyno.2009.10.043
20.
Thomas S Hussein Y Bandyopadhyay S Cote M Hassan O Abdulfatah E et al Interobserver variability in the diagnosis of uterine high-grade endometrioid carcinoma. Arch Pathol Lab Med (2016). 140(8):836–43. 10.5858/arpa.2015-0220-OA
21.
Wang X Nagl NG Wilsker D Van Scoy M Pacchione S Yaciuk P et al Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem J (2004). 383(Pt 2):319–25. 10.1042/BJ20040524
22.
Cancer Genome Atlas Research N Kandoth C Schultz N Cherniack AD Akbani R Liu Y et al Integrated genomic characterization of endometrial carcinoma. Nature (2013). 497(7447):67–73. 10.1038/nature12113
23.
McConechy MK Ding J Cheang MC Wiegand KC Senz J Tone AA et al Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol (2012). 228(1):20–30. 10.1002/path.4056
24.
Wiegand KC Lee AF Al-Agha OM Chow C Kalloger SE Scott DW et al Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol (2011). 224(3):328–33. 10.1002/path.2911
25.
Mao T-L Ardighieri L Ayhan A Kuo K-T Wu C-H Wang T-L et al Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am J Surg Pathol (2013). 37(9):1342–8. 10.1097/PAS.0b013e3182889dc3
26.
Werner HMJ Berg A Wik E Birkeland E Krakstad C Kusonmano K et al ARID1A loss is prevalent in endometrial hyperplasia with atypia and low-grade endometrioid carcinomas. Mod Pathol (2013). 26(3):428–34. 10.1038/modpathol.2012.174
27.
Jiang T Chen X Su C Ren S Zhou C . Pan-cancer analysis of ARID1A alterations as biomarkers for immunotherapy outcomes. J Cancer (2020). 11(4):776–80. 10.7150/jca.41296
28.
Kurz L Miklyaeva A Skowron MA Overbeck N Poschmann G Becker T et al ARID1A regulates transcription and the epigenetic landscape via POLE and DMAP1 while ARID1A deficiency or pharmacological inhibition sensitizes germ cell tumor cells to ATR inhibition. Cancers (2020). 12(4):905. 10.3390/cancers12040905
29.
Lee YH Heo J-h. Kim TH Kang H Kim G Kim J et al Significance of cell cycle regulatory proteins as malignant and prognostic biomarkers in ovarian epithelial tumors. Int J Gynecol Pathol (2011). 33(3):205–17. 10.1097/PGP.0b013e3182063e71
30.
Bosse T Nout RA McAlpine JN McConechy MK Britton H Hussein YR et al Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol (2018). 42(5):561–8. 10.1097/pas.0000000000001020
31.
Karamurzin Y Rutgers JKL . DNA mismatch repair deficiency in endometrial carcinoma. Int J Gynecol Pathol (2009). 28(3):239–55. 10.1097/PGP.0b013e31818d8fe6
32.
Garg K Shih K Barakat R Zhou Q Iasonos A Soslow RA . Endometrial carcinomas in women aged 40 years and younger: tumors associated with loss of DNA mismatch repair proteins comprise a distinct clinicopathologic subset. Am J Surg Pathol (2009). 33(12):1869–77. 10.1097/PAS.0b013e3181bc9866
33.
Shih KK Garg K Levine DA Kauff ND Abu-Rustum NR Soslow RA et al Clinicopathologic significance of DNA mismatch repair protein defects and endometrial cancer in women 40years of age and younger. Gynecol Oncol (2011). 123(1):88–94. 10.1016/j.ygyno.2011.06.005
34.
Grzankowski KS Shimizu DM Kimata C Black M Terada KY . Clinical and pathologic features of young endometrial cancer patients with loss of mismatch repair expression. Gynecol Oncol (2012). 126(3):408–12. 10.1016/j.ygyno.2012.05.019
35.
Black D Soslow RA Levine DA Tornos C Chen SC Hummer AJ et al Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. JCO (2006). 24(11):1745–53. 10.1200/JCO.2005.04.1574
36.
Zighelboim I Goodfellow PJ Gao F Gibb RK Powell MA Rader JS et al Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. JCO (2007). 25(15):2042–8. 10.1200/JCO.2006.08.2107
37.
Diaz-Padilla I Romero N Amir E Matias-Guiu X Vilar E Muggia F et al Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncology/Hematology (2013). 88(1):154–67. 10.1016/j.critrevonc.2013.03.002
Summary
Keywords
endometrioid endometrial carcinoma, grade 3, prognosis, ARID1A, p53, mismatch repair
Citation
Kato MK, Yoshida H, Tanase Y, Uno M, Ishikawa M and Kato T (2021) Loss of ARID1A Expression as a Favorable Prognostic Factor in Early-Stage Grade 3 Endometrioid Endometrial Carcinoma Patients. Pathol. Oncol. Res. 27:598550. doi: 10.3389/pore.2021.598550
Received
25 August 2020
Accepted
12 February 2021
Published
25 March 2021
Volume
27 - 2021
Edited by
Gabor Cserni, University of Szeged, Hungary
Updates
Copyright
© 2021 Kato, Yoshida, Tanase, Uno, Ishikawa and Kato.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayumi Kobayashi Kato, maykobay@ncc.go.jp
†ORCID:Mayumi Kobayashi Katoorcid.org/0000-0003-4065-0289Hiroshi Yoshidaorcid.org/0000-0002-7569-7813Yasuhito Tanaseorcid.org/0000-0002-3397-1482Masaya Unoorcid.org/0000-0001-8549-1635Mitsuya Ishikawaorcid.org/0000-0001-8855-2966Tomoyasu Katoorcid.org/0000-0001-9561-1522
‡These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.