Abstract
Immune checkpoint inhibitors (ICIs) have shown encouraging outcomes against Lynch syndrome (LS)-associated colorectal cancer (CRC) and endometrial cancer with mismatch repair deficient/microsatellite instability–high (dMMR/MSI-H). However, there is as yet no clarity on the safety and efficacy of immunotherapy combined with chemotherapy in LS-associated urothelial carcinoma (UC). Here, we report a patient with recurrent and metastatic LS-associated UC who achieved sustained response to programmed death protein 1 (PD-1) inhibitor combined with chemotherapy over 31 months, during which the side effects of immunotherapy could be controlled and managed. Our findings indicate that the dMMR/MSI status and PD-1 expression in UC may have potential predictive value for the response to PD-1-targeted immunotherapy. Our case supports the inclusion of such combination and/or monotherapy for UC in clinical studies and using dMMR/MSI status and PD-1 expression as potential predictive biomarkers for assessment of the therapeutic response.
Introduction
Urothelial carcinoma (UC) is a common malignant tumor that occurs in the bladder or upper urinary tract. In recent decades, surgery and combination chemotherapy demonstrate the improved efficacy of UC. However, a proportion of patients with advanced and metastatic UC after first-line and second-line treatment have a particularly poor prognosis [1, 2]. Several ongoing clinical trials in UC are exploring the potential survival benefit from immune checkpoint inhibitors (ICIs) targeting the programmed death protein 1 (PD1)/PD-L1 (ligand) pathway [3]. The utility of immunotherapy is expected to shape the future treatment of UC.
As one of the most common hereditary cancer predisposition syndromes, Lynch syndrome (LS) is caused by germline mutations in the DNA mismatch repair (MMR) genes, resulting in deficient MMR (dMMR). LS is associated with a markedly increased lifetime risk of colorectal cancer (CRC); endometrial cancer; cancers of the urothelial tract, stomach, ovary, pancreas, biliary tract; and sebaceous neoplasms of the skin. Recently, many studies have demonstrated that PD1/PD-L1 blockade therapies have profound implications for the medical management of LS-associated advanced CRC and endometrial cancer [4, 5]. However, reports of successful treatment of metastatic LS-associated UC using ICIs are limited. Here, we present a patient with LS who developed recurrent UC with lung/bone metastasis and displayed a favorable and sustained response to PD-1 inhibitors combined with chemotherapy. An additional observation worth discussing is the potential predictive biomarkers of clinical response to PD-1-targeted immunotherapy in UC.
Case Report
Clinical Course
A 62-year-old man suffering from gross hematuria was referred to Dongying People’s Hospital in March 2017. He was diagnosed with ureteral cancer and synchronous bladder cancer based on images, and underwent nephroureterectomy and transurethral resection of bladder tumor (TURBT). Invasive high-grade UC was confirmed on pathology examination. Additional instillation of pirarubicin (30 mg) weekly for 8 weeks was done after the operation. After that, three TURBT procedures were performed for recurrent bladder UC between May and November of 2017, during which intravesical instillations of Bacillus Calmette–Guérin (BCG) were carried out consistently.
On 1 March 2018, computed tomography (CT) showed bilateral multiple pulmonary nodules, with needle biopsy and pathology demonstrating metastatic UC. A solid nodule in the right posterior bladder wall was considered recurrent UC. During the next 4 months, he received six cycles of gemcitabine (1 g/m2, 1.6 g on day 1) plus cisplatin (75 mg/m2, 40 mg on days 1, 2, and 3, every 21 days). Magnetic resonance imaging (MRI) showed multiple bone metastases in the left proximal femur, pelvis, and left lower extremity bone. The patient underwent radiotherapy (95% PTV, 40 Gy/20f) for bone metastasis over 4 weeks. He also received six consecutive cycles of docetaxel (75 mg/m2, 120 mg on day 1, every 21 days) between 9 December 2018 and 13 March 2019, during which urinalysis displayed consistently high red blood cell (RBC) counts (Figure 1A). Imaging on 14 March 2019 revealed progressive bladder UC and aggravated bilateral lung metastasis and left femur metastasis (Figure 1B). Treatment response evaluation identified stable disease according to the response evaluation criteria for solid tumors. Moreover, gene testing of UC showed a microsatellite instability–high (MSI-H) phenotype. Accordingly, the patient was subsequently started on four cycles of the PD-1 inhibitor sintilimab (200 mg on day 1) plus docetaxel (120 mg on day 2, every 21 days) on 22 April 2019. After cycle two, a CT scan on 4 June 2019 revealed a disappeared nodule in the bladder, lung, and left proximal femur (Figure 1B). The patient had pruritus and maculopapular rash along the head and neck following sintilimab injection. Loratadine was effective against the allergic symptoms. He received the last sintilimab (200 mg on day 1) monotherapy on 30 July 2019. Two months later, the patient was again admitted to the hospital because of long-term fever and anorexia. Laboratory tests showed a free thyroxine level of 42 pmol/L, thyroid stimulating hormone 0.01 μIU/ml. Adrenocorticotropic hormone was assessed, which was <1 pg/ml (7.2–63.4 pg/ml) and hydroxycorticosteroid level 1.4 mg/24 h (2.0–10.0 mg/24 h), a morning cortisol level 8.4 nmol/L (172–497 nmol/L). Accordingly, the patient was diagnosed with sintilimab-associated hypophysitis and hypothyroidism. He was treated with thiamazole (10 mg, daily) and prednisone (5 mg at 8:00 a.m. and 2.5 mg at 4:00 p.m.). The patient’s symptoms improved significantly. Thereafter, thyroxine (0.2 mg) and prednisone (7.5 mg) were taken orally every day. According to the Common Terminology Criteria for Adverse Events v5.0 (https://www.gbg.de/de/rechner/ctcae.php), the patient’s symptoms met the criteria of Grade 2. Between 2020 and 2022, urinary RBC count was tested every 3 months and CT imaging of the chest/pelvis/bones was done every 6 months. Up to 30 March 2022, the patient remained symptom-free. Urinalysis (Figure 1A) and CT imaging (Figure 1B) showed a sustained response to therapy.
FIGURE 1
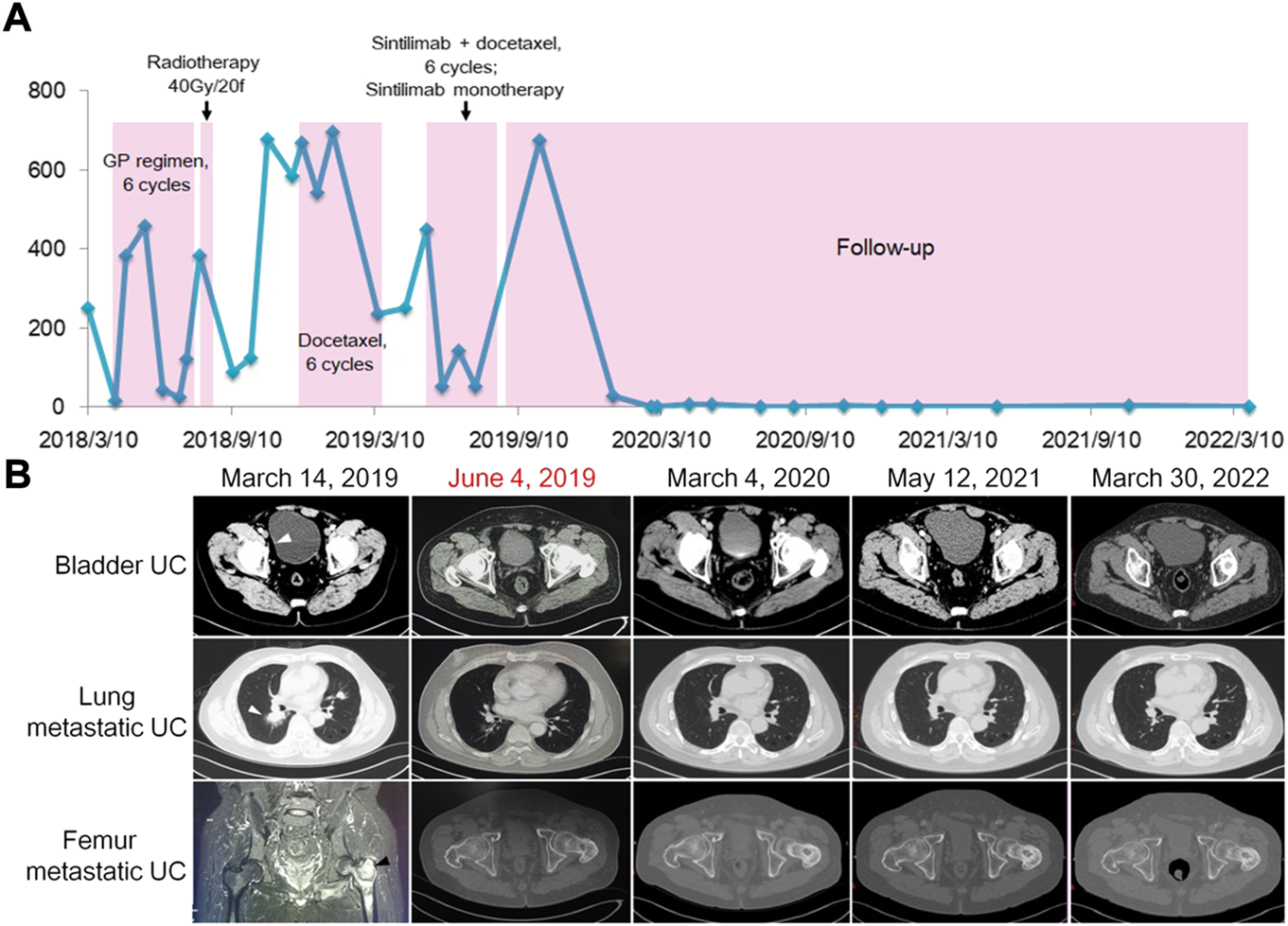
Clinical monitoring of treatment response in a patient with recurrent and metastatic UC. (A) Red blood cell count monitoring in urine. (B). Images of bladder UC (arrowhead) and metastatic lesions (arrowheads) before and after combined immunotherapy, exhibiting vanishing and stabilization of tumors.
Immunohistochemistry and Gene Testing
The patient had multiple metachronous cancers, including stomach cancer at age 34 years, colon cancers at ages 40 and 54 years, and skin cancer at age 66 years. We detected the expression of MMR proteins (MLH1: clone ES05, 1:100, PMS2: clone EP51, 1:50, MSH2: clone RED2, 1:100, and MSH6: clone EP49, 1:50; DAKO) in tumors using immunohistochemistry (IHC) staining. As shown in Figure 2A, loss of MSH2 and MSH6 expression was observed in UC, suggesting a dMMR status in UC.
FIGURE 2
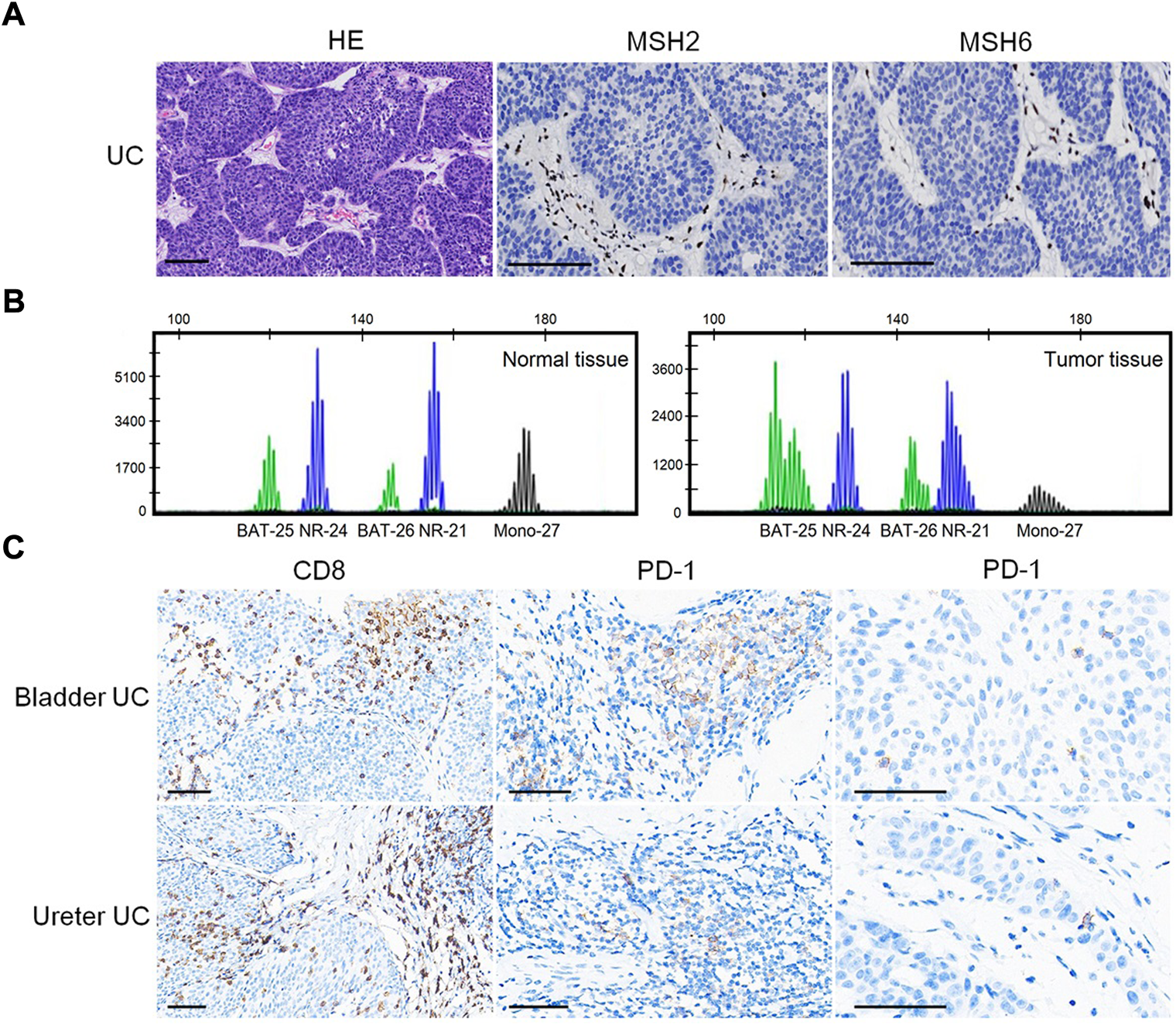
Predictive biomarkers for assessment of therapeutic response. (A) Loss of MSH2 and MSH6 expression in UC. Bar = 100 μm. (B) MSI analysis based on a pentaplex panel. (C) CD8 and PD-1 expression in UC. Bar = 50 μm.
We performed MSI gene locus assays with a pentaplex panel of five mononucleotides (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) using the Promega MSI Analysis System. The results showed four (BAT-25, BAT-26, NR-21, and MONO-27) loci with instability (Figure 2B), indicating an MSI-H status. IHC staining showed abundant CD8+ (clone SP16, 1:200; ZSGB-BIO, China) T-cell infiltration either at the tumor–stroma interface or within the tumor mass (Figure 2C). PD-1 (clone OTI4F10, 1:100, ZSGB-BIO) expression was identified mainly on immune cells (ICs) in the stroma of UC, as well as in scattered single tumor cells (TCs), with a combined positive score (CPS: number of PD-1 expressing cells TCs and ICs relative to the total number of TCs) = 8 (Figure 2C). However, no PD-L1 (clone 22C3, 1:250, DAKO) expression was identified on ICs or TCs (CPS = 0; figures not shown).
In genetic assessment using direct sequencing, we detected a heterozygous pathogenic germline mutation of c.715C>T (p.Gln239*) in MSH2 exon 4 (Figure 3A), which resulted in a premature stop codon and caused a truncated or absent MSH2 protein (Figure 3B). Accordingly, the patient was diagnosed with LS [6]. The pedigree chart of the family is presented in Figure 3C.
FIGURE 3
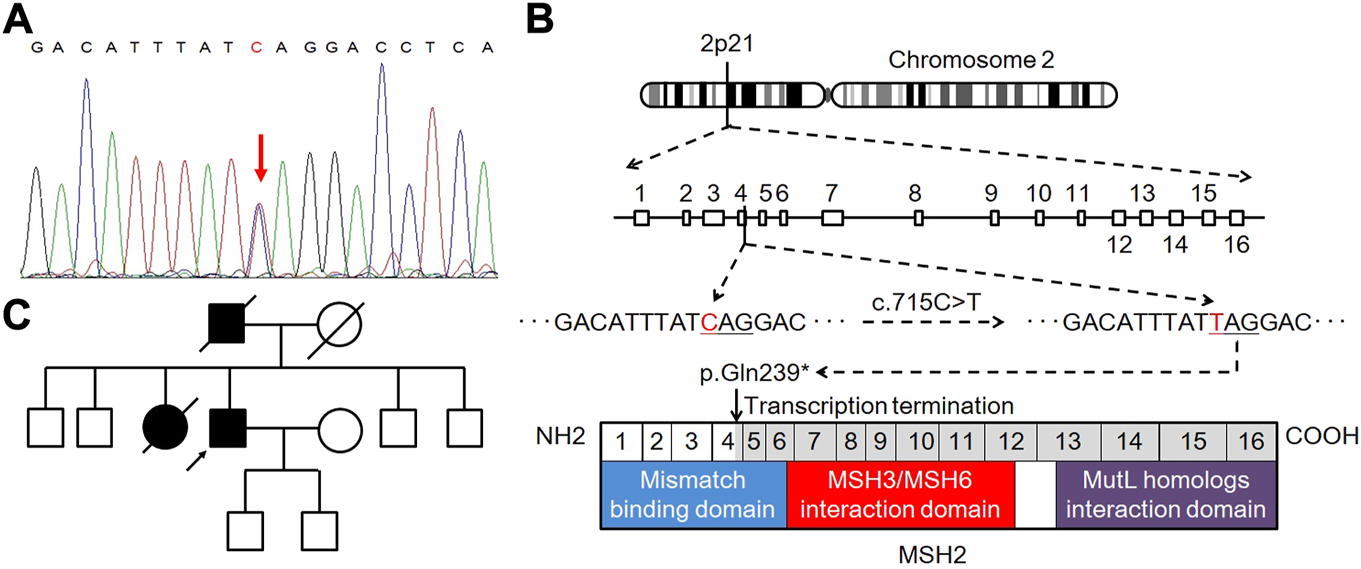
Genetic testing and pedigree analysis. (A) Heterozygous pathogenic germline mutation in the MSH2 gene of the proband. (B) Schematic representation of deleterious C.715C>T (p.Gln239*) mutation in MSH2 exon 4, leading to premature transcription termination and non-functional MSH2 protein product. (C) Pedigree chart of the family.
Discussion
Here, we report the case of a patient with recurrent and metastatic LS-associated UC who achieved a sustained response to PD-1 inhibitors combined with chemotherapy. Although the patient received cisplatin-based first-line chemotherapy and docetaxel second-line monotherapy, disease progression was detected, including local recurrence and lung/bone metastasis, suggesting limited benefit from chemotherapy. As a PD-1 inhibitor, sintilimab is currently approved in China but not in other countries for tumor immunotherapy. Sintilimab displays few adverse reactions and significant efficacy against a variety of solid tumors [7]. However, there are limited reports about the treatment of UC with PD-1 inhibitor therapy alone or combined with other chemotherapeutic drugs. A recent report demonstrated the partial response to sintilimab combined with nanoparticle albumin-bound-paclitaxel therapy for recurrent bladder UC and pelvic metastasis over an 11-month follow-up [8]. In the present report, our patient with recurrent and metastatic UC achieved sustained response after PD-1 inhibitor therapy combined with docetaxel therapy over 31 months. Hence, PD-1 inhibitor therapy combined with chemotherapy is a promising therapeutic strategy for patients with recurrent and metastatic UC whose disease progresses after first-line and second-line treatment.
UC is considered the third most common cancer in LS and is included in both the revised Bethesda Guidelines and the Amsterdam II criterion [9]. LS-associated upper tract urothelial cancer (UTUC) has a reported lifetime individual risk between 2.9% and 28%, conferring up to a 22-time greater risk in LS than in the general population [10]. The relative risk of bladder UC in LS is 12.3% for men and 2.6% for women, with MSH2 mutations and MSI-H seen in 86% and 85.7% of cases, respectively [11]. The present patient with a personal/familial history displayed germline MSH2 gene mutation, dMMR (MSH2/MSH6 deficiency), and MSI-H in UC. Accordingly, he was diagnosed with LS. dMMR in LS-associated tumors resulting from germline mutations of MMR genes increases the likelihood of acquiring somatic genetic mutations, particularly in short-tandem repeat sequences, leading to MSI. Previous case reports have also shown prolonged, complete remission at least 11 months in a patient with sporadic, high-grade dMMR UC of the renal pelvis, who received combined PD-L1 inhibitor immunotherapy [12]. Recently, clinical trials have confirmed the predictive value of dMMR/MSI status for PD-1-targeted inhibitor pembrolizumab therapy in patients with dMMR/MSI-H unresectable/metastatic CRC and advanced endometrial cancer [4, 5]. MSI-H has been detected in 21% of UTUC cases, 87% of which were dMMR [13]. The present patient with dMMR/MSI-H recurrent and metastatic UC obtained significant and durable clinical benefits from combination immunotherapy. These studies suggest the dMMR/MSI status in UC is a potential biomarker for predicting the response to immunotherapy. Moreover, dMMR/MSI status should be fully investigated in all UC cases, especially for patients who meet the Bethesda Guidelines and the Amsterdam II criteria.
Tumors with dMMR/MSI-H produce a plethora of immunogenic neoantigens, resulting in increased CD8+ lymphocyte infiltration and upregulation of genes encoding immune checkpoints, such as PD-1, PD-L1, and CTLA4 [14]. Although it is reported that dMMR is uncommon (2%) in muscle-invasive and high-grade UC of the bladder, the increased cytotoxic T lymphocytes and PD-L1 expression in these cases have been demonstrated [15]. Indeed, high versus low PD-L1 expression is associated with a greater likelihood of treatment benefit with anti-PD-L1 therapy in advanced or metastatic UC [16]. Companion PD-L1 IHC diagnostic assays are approved by the US Food and Drug Administration (FDA) for use in patients with advanced UC. In the present patient, PD-L1 expression not detected on TCs or on ICs. However, we observed more PD-1 expression on ICs than on TCs, which suggested the potential predictive value of PD-1 expression in the response to immunotherapy. However, larger studies are required to fully investigate PD-1 expression in all UC cases and treatment benefits from PD-1/PD-L1 blockade therapy. Also, scoring algorithms and validation metrics of PD-1 IHC tests require more research in the future.
In conclusion, we describe a patient with recurrent and metastatic LS-associated UC who achieved a sustained response to PD-1 inhibitors combined with chemotherapy over 31 months, during which the side effects of immunotherapy could be controlled and managed. Our case supports the inclusion of such combination and/or monotherapy in clinical studies and using dMMR/MSI-H status and PD-1 expression as potential predictive markers for the assessment of therapeutic response.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Consent obtained directly from patient. This study was approved by the institutional review board of Shandong Provincial Hospital (SZRJJ: NO. 2021-213).
Author contributions
Y-TM, H-LY, and D-GW analyzed the data and drafted the manuscript; H-LY and YL provided patient care; D-GW, Y-JQ, and M-QZ conducted pathological examination; LY, G-YX, DS, and HN provided clinical assistance; Y-TM and Y-JX performed immunohistochemistry and gene testing; Z-GY and FH designed the study.
Funding
This study was supported by the Natural Sciences Foundation of Shandong Province (ZR2021MH095), Beijing CSCO Clinical Oncology Research Foundation (Y-tongshu2021/ms-0163), and General program of National Natural Science Foundation of China (82071035).
Acknowledgments
The authors would like to thank the patient and his family members who provided the data on clinical treatments. The authors would also like to thank the surgeons, physicians, nurses, research coordinators, and other staff at the hospital who assisted with the study. The authors would like to acknowledge the Biosune Biotechnology (Shanghai) team for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
De Santis M Bellmunt J Mead G Kerst JM Leahy M Maroto P et al Randomized Phase II/III Trial Assessing Gemcitabine/carboplatin and Methotrexate/carboplatin/vinblastine in Patients with Advanced Urothelial Cancer Who Are Unfit for Cisplatin-Based Chemotherapy: EORTC Study 30986. J Clin Oncol (2012) 30:191–9. 10.1200/JCO.2011.37.3571
2.
Galsky MD Pal SK Lin SW Ogale S Zivkovic M Simpson J et al Real-World Effectiveness of Chemotherapy in Elderly Patients with Metastatic Bladder Cancer in the United States. Bladder Cancer (2018) 4:227–38. 10.3233/BLC-170149
3.
Rhea LP Mendez-Marti S Kim D Aragon-Ching JB . Role of Immunotherapy in Bladder Cancer. Cancer Treat Res Commun (2021) 26:100296. 10.1016/j.ctarc.2020.100296
4.
Andre T Shiu KK Kim TW Jensen BV Jensen LH Punt C et al Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med (2020) 383:2207–18. 10.1056/NEJMoa2017699
5.
O'Malley DM Bariani GM Cassier PA Marabelle A Hansen AR De Jesus Acosta A et al Pembrolizumab in Patients with Microsatellite Instability-High Advanced Endometrial Cancer: Results from the KEYNOTE-158 Study. J Clin Oncol (2022) 40:752–61. 10.1200/JCO.21.01874
6.
Skeldon SC Semotiuk K Aronson M Holter S Gallinger S Pollett A et al Patients with Lynch Syndrome Mismatch Repair Gene Mutations Are at Higher Risk for Not Only Upper Tract Urothelial Cancer but Also Bladder Cancer. Eur Urol (2013) 63:379–85. 10.1016/j.eururo.2012.07.047
7.
Huang N Zhao C Hu X Zhang C Xiong F Huang W et al Safety and Efficacy of Sintilimab Combination Therapy for the Treatment of 48 Patients with Advanced Malignant Tumors. Transl Cancer Res (2022) 11:252–61. 10.21037/tcr-22-54
8.
Cao JZ Wu W Pan JF Wang HW Jiang JH Ma Q . Case Report: Anlotinib Combined with Sintilimab as Third-Line Treatment in a Metastatic Urothelial Bladder Carcinoma Patient with FGFR3 Mutation. Front Oncol (2021) 11:643413. 10.3389/fonc.2021.643413
9.
Umar A Boland CR Terdiman JP Syngal S de la Chapelle A Ruschoff J et al Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J Natl Cancer Inst (2004) 96:261–8. 10.1093/jnci/djh034
10.
Huang D Matin SF Lawrentschuk N Roupret M . Systematic Review: An Update on the Spectrum of Urological Malignancies in Lynch Syndrome. Bladder Cancer (2018) 4:261–8. 10.3233/BLC-180180
11.
van der Post RS Kiemeney LA Ligtenberg MJ Witjes JA Hulsbergen-van de Kaa CA BoDmer D et al Risk of Urothelial Bladder Cancer in Lynch Syndrome Is Increased, in Particular Among MSH2 Mutation Carriers. J Med Genet (2010) 47:464–70. 10.1136/jmg.2010.076992
12.
Castro MP Goldstein N . Mismatch Repair Deficiency Associated with Complete Remission to Combination Programmed Cell Death Ligand Immune Therapy in a Patient with Sporadic Urothelial Carcinoma: Immunotheranostic Considerations. J Immunother Cancer (2015) 3:58. 10.1186/s40425-015-0104-y
13.
Hartmann A Zanardo L Bocker-Edmonston T Blaszyk H Dietmaier W Stoehr R et al Frequent Microsatellite Instability in Sporadic Tumors of the Upper Urinary Tract. Cancer Res (2002) 62:6796–802.
14.
Teo MY Seier K Ostrovnaya I Regazzi AM Kania BE Moran MM et al Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit from PD-1/pd-L1 Blockade in Advanced Urothelial Cancers. J Clin Oncol (2018) 36:1685–94. 10.1200/JCO.2017.75.7740
15.
Hodgson A Vesprini D Liu SK Xu B Downes MR . Correlation of Mismatch Repair Protein Deficiency, PD-L1 and CD8 Expression in High-Grade Urothelial Carcinoma of the Bladder. J Clin Pathol (2020) 73:519–22. 10.1136/jclinpath-2019-206256
16.
Massard C Gordon MS Sharma S Rafii S Wainberg ZA Luke J et al Safety and Efficacy of Durvalumab (MEDI4736), an Anti-programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients with Advanced Urothelial Bladder Cancer. J Clin Oncol (2016) 34:3119–25. 10.1200/JCO.2016.67.9761
Summary
Keywords
immunotherapy, microsatellite instability, immune checkpoint inhibitors, urothelial carcinoma, lynch syndrome, PD-1/PD-L1
Citation
Ma Y-T, Li Y, Yan L, Hua F, Wang D-G, Xu G-Y, Yang H-L, Xue Y-J, Qin Y-J, Sha D, Ning H, Zhao M-Q and Yao Z-G (2022) Case Report: Potential Predictive Value of MMR/MSI Status and PD-1 Expression in Immunotherapy for Urothelial Carcinoma. Pathol. Oncol. Res. 28:1610638. doi: 10.3389/pore.2022.1610638
Received
04 June 2022
Accepted
11 October 2022
Published
21 October 2022
Volume
28 - 2022
Edited by
József Tímár, Semmelweis University, Hungary
Updates
Copyright
© 2022 Ma, Li, Yan, Hua, Wang, Xu, Yang, Xue, Qin, Sha, Ning, Zhao and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Gang Yao, yzg20062009@163.com; Miao-Qing Zhao, zhaomqsd@163.com
†These authors have contributed equally to this work
‡ORCID: Zhi-Gang Yao, orcid.org/0000-0002-8034-6524
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.