Abstract
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is increasingly performed in patients with advanced cancer in the abdomen. This treatment prolongs survival for some patients but is known to have a substantial rate of complications. Choosing patients for this procedure can be difficult, and no clear guidelines exist. Muscle mass is a general measure of a patient’s wellness, meaning that patients with low muscle mass for their body weight tend to have more complications from treatment and overall do worse. We evaluated muscle mass prior to surgery in our Cytoreductive surgery/hyperthermic intraperitoneal chemotherapy population to assess how many patients have low muscle mass and the impact on outcomes, such as length of hospital stay, complications and survival. We find that about 25% of our patient population has low muscle mass, and low muscle mass is associated with a higher burden of cancer and shorter survival. We were able to evaluate muscle mass in a small number of patients after surgery, expecting to find decreased muscle mass in all the patients after a complex operation and long recovery. In fact, none of the patients had low muscle mass, including those who were low prior to surgery.
Introduction
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are increasingly performed for patients with peritoneal spread of gastrointestinal (GI) and gynecologic (GYN) malignancies and mesothelioma. Although this procedure has provided prolonged survival for patients, it is associated with a relatively high complication rate. Patient selection remains critical for success of this operation, but current selection criteria are either not oncologic (i.e. performance status, albumin level) or not modifiable (i.e. peritoneal cancer index (PCI), histology). We sought to determine an improved marker of patient outcome as well as a parameter for which intervention was possible.
Sarcopenia is degenerative loss of skeletal muscle mass. It is associated with decreased survival in the general population, and has also been associated with poor outcomes in patients with localized colorectal cancer, hepatocellular cancer, pancreatic cancer and women with ovarian cancer. Patients with sarcopenia have been found to have increased toxicity from intraperitoneal chemotherapy [1], and sarcopenic patients with colon cancer have poor outcomes with CRS-HIPEC [2]. We evaluated our HIPEC patient population to determine the frequency of sarcopenia, and assess for any association with length of stay (LOS), complications or post-HIPEC survival.
Methods
We performed a retrospective review of consecutive HIPEC patients at our institution from 11/2013–4/2018, with imaging available for analysis within 2 months prior to CRS/HIPEC. Multiple factors are considered when selecting patients for CRS/HIPEC including the origin and histology of the tumor, burden of disease, underlying medical problems and fitness for surgery, and extent and response to systemic agents. In uncertain cases we may perform diagnostic laparoscopy first to assess resectability. For patients who had 2 CRS/HIPEC procedures we utilized data from the first procedure only. Demographic variables including age, diagnosis, date of diagnosis, BMI, serum albumin, chemotherapy prior to HIPEC, postoperative complications, length of stay (LOS) and post-HIPEC survival were recorded. The burden of disease is routinely assessed for each patient with the Peritoneal Cancer Index (PCI), a standardized score [3] in which the largest tumor nodule in each of 13 different sections of the abdomen is measured. No disease is scored as 0, disease measuring up to 0.5 cm is scored as 1, up to 5 cm scored as two and >5 cm or confluent disease as 3. The PCI can range from 0–39. The Aquarius iNtuition imaging calculation software (TeraRecon, Durham, NC) [4] was used to assess the CT scans and calculate skeletal muscle mass as well as subcutaneous and visceral fat to evaluate for sarcopenia [5] (Figure 1). Sarcopenia was defined as skeletal muscle mass <52 cm2/m2 for normal or overweight men and <54 cm2/m2 for obese men, <38 cm2/m2 for normal or overweight women and <47 cm2/m2 for obese women [6]. Obesity is defined as a BMI of 30 or greater. Post-HIPEC CT scans are performed between 3 and 6 months after surgery to initiate surveillance, or as indicated to evaluate for complications. Patients with imaging between 2 and 4 months after HIPEC were evaluated for postoperative muscle mass.
FIGURE 1
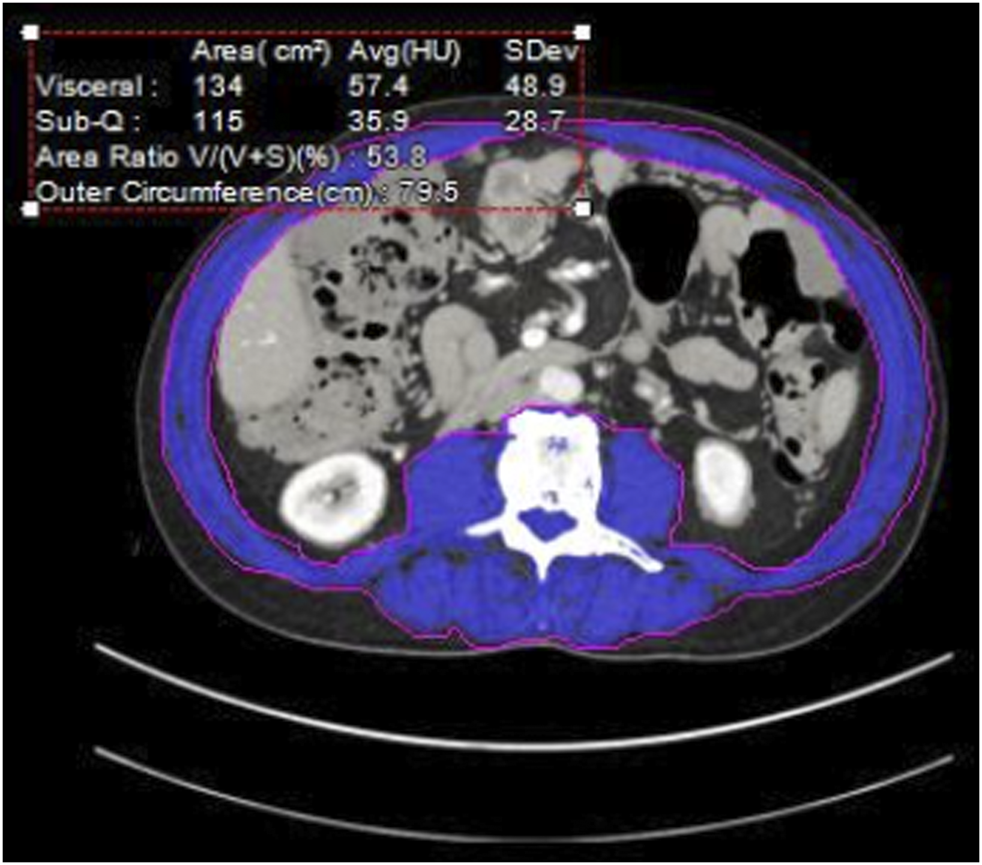
Cross sectional Abdominal CT at L3. Blue area—quantified muscle [3].
We compared outcomes and demographic variables by sarcopenia status using Wilcoxon rank-sum tests for continuous variables and chi-squared tests for categorical variables. Post-HIPEC survival was defined as the number of days from the date of surgery to the date of death. Patients who were still alive at the time of analysis were considered censored. The method of Kaplan and Meier [7] was used to estimate survival curves by sarcopenia status, and the logrank test was used to test for differences between sarcopenia groups. Our protocol was approved by the Penn State Institutional Review Board, STUDY9023. Informed consent was waived for this study due to the retrospective nature of the study and the high mortality rate of the patient population.
Results
We identified 89 patients eligible for review and found that 25% had sarcopenia. Sarcopenia was associated with a higher PCI, median of 19 vs. 14 (p = 0.06) and shorter overall survival (Figure 2), median of 1.5 years vs. not reached by 4 years (p = 0.003), but sarcopenia did not predict a lower rate of complete cytoreduction, with 59 vs. 62% having a CC0/1 cytoreduction, p = 0.9. Our patients with sarcopenia had a statistically significantly lower BMI than the non-sarcopenic patients, 25.7 vs. 29.3, p = 0.002, yet their BMI was within normal range (Table 1). A majority of patients did not have weight loss prior to the scan. There was no difference in post-operative complication rate, age, gender or serum albumin level between sarcopenic and non-sarcopenic patients. Sarcopenic patients were more likely to have received chemotherapy, 77 vs. 58%, although this was not statistically significant, p = 0.11, and a longer length of stay, 10 vs. 8 days, although this was also not statistically significant (p = 0.12). The findings were similar when including the entire HIPEC population or selecting for appendiceal and colorectal cancer only. We identified 18 patients with CT scans between 2 and 4 months after HIPEC, with five of the 18 patients being sarcopenic prior to HIPEC. Post HIPEC muscle mass evaluation demonstrated that the non-sarcopenic patients remained non-sarcopenic. Only one of the sarcopenic patients remained sarcopenic, the other four patients had improved muscle mass and were within normal range. The four patients with sarcopenia resolution had an average post-HIPEC survival of 2.47 years (945 days) compared to the remaining sarcopenic patients, who had an average post-HIPEC survival of 1.09 years (401 days).
FIGURE 2
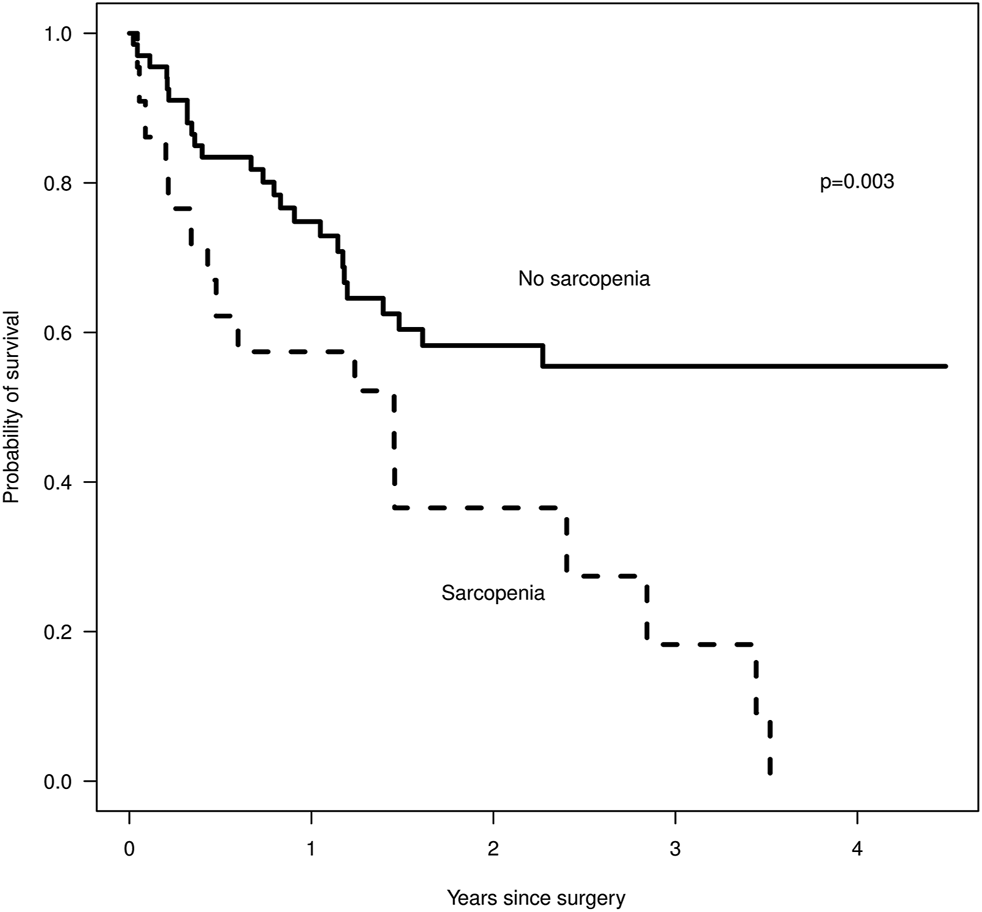
Survival after surgery by sarcopenia status, Kaplan-Meier, where n = 22 for the sarcopenic group and n = 67 for the non-sarcopenic group.
TABLE 1
| Sarcopenic n = 22 | Non-sarcopenic n = 67 | p Value | |
|---|---|---|---|
| BMI, median (IQR) | 25.7 (21.6–28.1) | 29.3 (24.6–33.2) | 0.002 |
| PCI, median (IQR) | 19 (11–26) | 14 (6–24) | 0.06 |
| LOS, days, median (IQR) | 10 (7–11) | 8 (6–10) | 0.12 |
| Sys chemo*, N (%) | 17 (77%) | 39 (58%) | 0.11 |
| Age, median (IQR) | 57 (20–77) | 58 (25–82) | 0.82 |
| Sex, female, N (%) | 11 (50%) | 38 (57%) | 0.58 |
| Duration of disease#, median (IQR) | 10.6 (7.3–20.3) | 11.5 (2.6–28.3) | 0.95 |
| Albumin, median (IQR) | 3.7 (3.1–4.3) | 4.1 (3.4–4.4) | 0.32 |
| Primary Tumor: | |||
| Colon, N (%) | 9 (41%) | 26 (39%) | 0.86 |
| Appendix, N (%) | 7 (32%) | 22 (33%) | 0.93 |
| Lamn, N (%) | 2 (9%) | 3 (4%) | 0.41 |
| Ovary, N (%) | 1 (5%) | 5 (7%) | 0.64 |
| Other^, N (%) | 3 (14%) | 11 (16%) | 0.76 |
Comparison of sarcopenic and non-sarcopenic patients.
*Any systemic chemotherapy during 1 year prior to scan. #Time from diagnosis to CRS/HIPEC in months. ∧Gallbladder, gastric, desmoplastic small round cell, endometrium, rectum, mesothelioma, unknown primary, pancreas.
IQR, Interquartile range; BMI, body mass index; PCI, peritoneal cancer index; LOS, length of stay; LAMN, low grade mucinous neoplasm of appendix.
Discussion
Sarcopenia is defined as low skeletal muscle mass and is conventionally measured on cross-sectional imaging at the level of the third lumbar vertebrae. Many factors can be associated with sarcopenia including age, malignancy, co-morbidities, gender and malnutrition [8, 9]. Sarcopenia has been associated with decreased survival time in patients with stage 1–3 colorectal cancer [6] and lower disease free and overall survival rates after resection of colorectal liver metastases [10]. Patients undergoing CRS/HIPEC for colorectal cancer metastases have an increased risk of severe postoperative complications [2] and increased toxicity from the intraperitoneal chemotherapy [1] if they are sarcopenic, making sarcopenia a relevant marker in this patient population for both short- and long-term poor outcomes.
Sarcopenia was present in 25% of our HIPEC patients and was an indication of a higher burden of disease and shorter survival time. The rate of sarcopenia was lower than described in other series [2, 6, 11], although patients who are candidates for CRS/HIPEC represent a highly select group of patients with metastatic disease. Nutritional parameters such as serum albumin level are often used to predict patient outcome [12] and the risk of complications, however in this patient population there was no difference between groups, and the sarcopenic patients had an albumin level considered within normal range. Similarly, the sarcopenic patients had a BMI within normal range, and no history of recent weight loss. These findings are consistent with other reports and have several important implications. The presence of sarcopenia may not be clinically apparent and all patients should have muscle mass calculated. The presence of sarcopenia has significant consequences for patient outcome and may help in counseling and selecting patients for surgery.
Patients who received systemic therapy prior to HIPEC were more likely to be sarcopenic, although this did not reach statistical significance likely due to the small number of patients in this study. With the wide variety of tumor types and timing of presentation for HIPEC there was significant variability in the chemotherapy regimens and duration of treatment. For this small number of patients we elected to separate them into “any chemotherapy” and “no chemotherapy” groups. Previous work has demonstrated that a high percentage of patients will have loss of skeletal muscle mass during chemotherapy treatment [13], and sarcopenia is associated with higher rates of treatment related complications and inability to complete prescribed chemotherapy regimens [14]. Strategies to maintain muscle mass can lead to improved tolerance of treatment [15] and reduced side effects [16]. A majority of patients who are candidates for CRS/HIPEC will be eligible for and receive systemic therapy at some point during their treatment. While the impact of muscle mass on treatment related complications is clear, it is unknown whether treatment planning based on muscle mass will improve outcome. For example, a patient with sarcopenia may have a better outcome with CRS/HIPEC first followed by systemic therapy rather than systemic therapy first.
The improvement in muscle mass after HIPEC was unexpected, particularly among the patients who already had sarcopenia. We anticipated further loss of muscle in the short term after the stress of a long operation and intraperitoneal chemotherapy. The number of patients is too small to draw statistically significant conclusions, but the finding suggests that elimination of the burden of cancer may improve a patient’s physiology. Alternatively, those patients who are physiologically able to build muscle mass after CRS/HIPEC may represent the select group of patients with better disease biology. In this study we chose to focus on short-term muscle mass and therefore did not evaluate scans more than 4 months after HIPEC. Additional longitudinal studies that measure muscle mass over the entire course of a patient’s treatment should be performed.
Sarcopenia may be an indicator of more advanced systemic disease, a longer duration of treatment or both. It is not well documented in the literature if sarcopenia can be reversed in this patient population, or if addressing sarcopenia can alter patient outcomes. Further studies are needed in order to better understand the effect of sarcopenia on this patient population, including interventional trials that attempt to reverse loss of muscle mass. Our study is limited in its retrospective nature as well as the small sample size. Additionally, not all patients received CT scans in the 2–4 months following their HIPEC procedure thus reducing our sample size further for post HIPEC sarcopenia rate. It is possible that this biased our results in finding that only 1/5 patients continued to have sarcopenia following HIPEC.
Conclusion
Sarcopenia is present in patients with advanced malignancy and may be clinically occult. The presence of sarcopenia is associated with worse short and long-term outcomes including survival. Larger studies are needed in this patient population and interventional trials should be considered.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board, The Pennsylvania State University, College of Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study concept and design: CP Acquisition and analysis of data: AG, AJ, and ES. Interpretation of data: ES and CP Drafting of the manuscript: CP, AJ, and BS. Critical revision of the manuscript for important intellectual content: CP. Study supervision: CP.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Chemama S Bayar MA Lanoy E Ammari S Stoclin A Goéré D et al Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol (2016). 23(12):3891–8. 10.1245/s10434-016-5360-7
2.
van Vugt JLA Braam HJ van Oudheusden TR Vestering A Bollen TL Wiezer MJ et al Skeletal muscle depletion is associated with severe postoperative complications in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol (2015). 22(11):3625–31. 10.1245/s10434-015-4429-z
3.
Jacquet P Sugarbaker PH . Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res (1996). 82:359–74. 10.1007/978-1-4613-1247-5_23
4.
Lodewick TM Arnoldussen CWKP Lahaye MJ van Mierlo KMC Neumann UP Beets-Tan RG et al Fast and accurate liver volumetry prior to hepatectomy. Hpb (2016). 18(9):764–72. 10.1016/j.hpb.2016.06.009
5.
Chung E Lee HS Cho E-S Park EJ Baik SH Lee KY et al Prognostic significance of sarcopenia and skeletal muscle mass change during preoperative chemoradiotherapy in locally advanced rectal cancer. Clin Nutr (2020). 39(3):820–8. 10.1016/j.clnu.2019.03.014
6.
Cespedes Feliciano EM Kroenke CH Meyerhardt JA Prado CM Bradshaw PT Kwan ML et al Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol (2017). 3(12):e172319. 10.1001/jamaoncol.2017.2319
7.
Kaplan EL Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc (1958). 53:457–81. 10.1080/01621459.1958.10501452
8.
Mayhew AJ Amog K Phillips S Parise G McNicholas PD de Souza RJ et al The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing (2019). 48(1):48–56. 10.1093/ageing/afy106
9.
Hanach NI McCullough F Avery A . The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv Nutr (2019). 10(1):59–69. 10.1093/advances/nmy065
10.
van Vledder MG Levolger S Ayez N Verhoef C Tran TCK Ijzermans JNM . Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg (2012). 99(4):550–7. 10.1002/bjs.7823
11.
Lieffers JR Bathe OF Fassbender K Winget M Baracos VE . Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer (2012). 107(6):931–6. 10.1038/bjc.2012.350
12.
Gupta D Lis CG . Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J (2010). 9(9), 27. 10.1186/1475-2891-9-69
13.
Choi Y Oh DY Kim TY Lee KH Han SW Im SA et al Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One (2015). 10(10):e0139749. 10.1371/journal.pone.0139749
14.
Xiao DY Luo S O'Brian K Ganti A Riedell P Sanfilippo KM et al Impact of sarcopenia on treatment tolerance in United States veterans with diffuse large B-cell lymphoma treated with CHOP-based chemotherapy. Am J Hematol (2016). 91(10):1002–7. 10.1002/ajh.24465
15.
Pin F Couch ME Bonetto A . Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care (2018). 12(4):420–6. 10.1097/spc.0000000000000382
16.
Zimmer P Trebing S Timmers-Trebing U Schenk A Paust R Bloch W et al Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer (2018). 26(2):615–24. 10.1007/s00520-017-3875-5
Summary
Keywords
sarcopenia, muscle mass, cytoreduction, outcomes, intraperitoneal chemotherapy, peritoneal surface disease
Citation
Juris A, Taylor-Gehman A, Spencer B, Schaefer E and Pameijer C (2021) The Impact of Sarcopenia in Patients with Peritoneal Surface Disease. Pathol. Oncol. Res. 27:638857. doi: 10.3389/pore.2021.638857
Received
07 December 2020
Accepted
01 March 2021
Published
30 August 2021
Volume
27 - 2021
Edited by
Andrea Ladányi, National Institute of Oncology (NIO), Hungary
Updates
Copyright
© 2021 Juris, Taylor-Gehman, Spencer, Schaefer and Pameijer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colette Pameijer, cpameijer@pennstatehealth.psu.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.