Abstract
Objective: No assessment was conducted describing the age and gender specific epidemiology of lung cancer (LC) prior to 2018 in Hungary, thus the objective of this study was to appraise the detailed epidemiology of lung cancer (ICD-10 C34) in Hungary based on a retrospective analysis of the National Health Insurance Fund database.
Methods: This longitudinal study included patients aged ≥20 years with LC diagnosis (ICD-10 C34) between January 1, 2011 and December 31, 2016. Patients with different cancer-related codes 6 months before or 12 months after LC diagnosis or having any cancer treatment other than lung cancer protocols were excluded.
Results: Lung cancer incidence and mortality increased with age, peaking in the 70–79 age group (375.0/100,000 person-years) among males, while at 60–69 age group for females (148.1/100,000 person-years). The male-to-female incidence rate ratio reached 2.46–3.01 (p < 0.0001) among the 70–79 age group. We found 2–11% decrease in male incidence rate at most age groups, while a significant 1–3% increase was observed in older females (>60) annually during the study period.
Conclusion: This nationwide epidemiology study demonstrated that LC incidence and mortality in Hungary decreased in younger male and female population, however we found significant increase of incidence in older female population, similar to international trends. Incidence rates peaked in younger age-groups compared to Western countries, most likely due to higher smoking prevalence in these cohorts, while lower age LC incidence could be attributed to higher competing cardiovascular risk resulting in earlier mortality in smoking population.
Introduction
Lung cancer was considered globally as a rare disease until the sharp rise starting from the 1930–40’s in the Western world, that culminated in lung cancer becoming the leading cause of preventable cancer -related death among men by the mid-century, with a notable increase in incidence in the female population as well during the past decades [1], showing a strong positive correlation with its main risk factor, the increase of smoking prevalence [2]. According to Cancer Research United Kingdom, a national cancer information portal, 79% of the incident cases in 2015 could have been prevented with effective health promotion interventions, thus avoiding significant financial and societal burden [3].
Numerous publications have found that lung cancer incidence shows a strong correlation with age, the highest incidence rates are found in elderly people [4]. The United Kingdom Cancer Research portal reported that between 2013 and 2015, more than 40% of incident lung cancer cases were diagnosed in the ≥75 years age group [5]. A recent Canadian study from 2015 by Akhtar-Danesh and colleagues, reported diverse trends for lung cancer incidence depending on age and sex: an increasing incidence was observed in elderly female age groups, whereas a decreasing trend in incidence was found among younger women and in most male age cohorts. Age-specific incidence rates started to increase in the age group of 45–49 years, with the highest rates found in the age group of 85–89 years in men and 80–84 years in women [6]. A study by the American Cancer Society from 2016 found that 53% of cases occur in individuals 55–74 years old and 37% occur over 75 years old in the United States. The highest incidence of lung cancer in men is 585.9 per 100,000 in 85–89 years old, while the highest incidence in women is 365.8 per 100,000 person years (PSYs) in 75–79 years old [7]
Previous publications also found differing trends in lung cancer incidence among male and female patients. A study from 2015 examining European cancer trends reported a significant, 12% decrease in the incidence of lung cancer among European men from 2009 to 2015, while an increase of 9% was found among women in the same time period, concluding that lung cancer incidence rates may exceed that of breast cancer in some EU countries by late 2010s’ [8]. Studies from Europe also concluded that in Western countries, men reached the peak of incidence and mortality for lung cancer, showing decreasing trends, with women approaching the plateau. It is mostly established that these trends are influenced by lifestyle, environment and chiefly, smoking habit rather than biological differences associated with gender [9,10].
Hungary is frequently reported having one of the highest incidence and mortality rates of lung cancer in Europe, though in a recent publication, these rates were revised, using a novel methodology based on NHIF database, thus significantly lower rates were found [11]. In recent years, no comprehensive, age and gender specific lung cancer appraisal was conducted in Hungary. Consequently, the primary goal of our study was to determine the age and gender specific incidence and mortality of lung cancer in Hungary between 2011 and 2016, and to assess the trends in both genders by age cohorts.
Materials and Methods
Study Design
The National Health Insurance Fund of Hungary (NHIF) provided the input data used for the in-depth analysis in this retrospective, longitudinal study covering lung cancer patients for the 6-years time period between 2011 and 2016. The NHIF is the principal public-healthcare financing agency in Hungary, with its database covering all patients and the entire care continuum for oncology treatments, with the exception of less common private care visits which are outside the scope of this study. The database thus represents nearly 100% of the Hungarian population (9,957,731 people covered – Hungarian Central Statistician Office (CSO) data, 2012), collecting ID and ICD-10 code information from all in- and out-patient visits, as well the entirety of prescription drugs reimbursed in Hungary. The study license number is I043/88/2019 (at NHIF), and the study ethical approval number issued by the Central Ethical Committee of Hungary is 10338–5/2019/EKU.
Lung cancer patients (ICD-10 C34) who were diagnosed between January 1, 2011 and December 31, 2016 were included in the study, if they were ≥20 years of age at the time of diagnosis. In order to minimize the risk of miscoding lung cancer, patients were included with a minimum of two incidences of the ICD-10 code C34 within more than 30 but less than 365 days. One occurrence of C34 was also accepted if a patient deceased within 60 days after the first C34 code was registered. Patients with different cancer-related codes 6 months before or 12 months after LC diagnosis or having any cancer treatment other than lung cancer protocols were excluded. A period of 3 years between 2008 and 2010 was considered as a reference period to detect newly diagnosed lung cancer patients in 2011. Each patient was tracked until December 31, 2016 or until the time of death. All data were anonymized prior to data extraction stage, ensuring that only non-identifiable data were used for further analyses. The size of relevant Hungarian populations used for incidence and prevalence calculations were obtained from the publicly available annual reports of the Hungarian CSO [12], while mortality data for the lung cancer population was obtained from the source NHIF database.
The annual numbers of newly diagnosed lung cancer patients are presented as crude numbers (n) by age cohorts. Age specific incidence and mortality rates are expressed as rates per 100,000 person-years. All-cause mortality was expressed as crude numbers by age cohorts as well as age-specific rates per 100,000 person-years. In order to allow for comparison with earlier publications, the European Standard Population (ESP) 2013 [13] age groups weights were used for standardization. The sign “<10” was used for raw numbers below 10, as data protection rules of Hungary forbids their publication. All calculations were run on the exact numbers. The following age- cohorts were used for analysis: 20–39; 40–49; 50–59; 60–69; 70–79, 80–89 and ≥90 (expect expressing detailed age specific rates in Supplementary Tables, where we used 20–29 and 30–39 cohorts instead of 20–39).
Statistical Analysis
Annual trends were estimated with 95% confidence intervals (95% CI). A block-based bootstrap method, with a fixed block size of 2 was used for time series, as data were not independent. Size of population at risk was calculated by subtracting the number of previously diagnosed lung cancer patients on 1st of January from mid-year population, for each study year. Linear regression was applied to calculate the annual change of the mean age of patients. The outcome was age in years, the explanatory variable was the year. The annual odds ratio of the proportion of males among lung cancer patients were calculated by binomial logistic regression. The outcome was the proportion of males, the explanatory variable was the year. Poisson regression was used to estimate crude incidence and mortality numbers, age-specific incidence and mortality rates, and incidence and mortality rate ratios. The outcome was the number of patients, the offset was the log of the number of patients at risk or the mid-year population, the explanatory variables were the year, age group, gender and their paired interactions. Incidence rate ratios (IRR) were calculated using contrasts. Independent linear models were created for the subgroups (sex, age groups) of each line of the Supplementary Tables. The only explanatory variable was the year. For all males, females and the whole patient population, the outcomes were the rates for 100,000 person-years standardized for the ESP 2013. All calculations were performed with R software, version 3.5.2 (2018-12-20) with package boot version 1.3-20.
Results
Crude Numbers
In 2011, 4,522 new male lung cancer cases were registered in the NHIF database, while we found 4,176 incident patients in 2016. The number of female patients increased from 2,636 to 2,828 during the same period (Table 1). Proportion of male patients decreased from 63.17% to 56.69% by the end of the study period. The mean age at diagnosis was 64.51 years for men (SD ± 9.85) and 64.93 years for women (SD ± 11.19) in 2011, increasing steadily to 65.80 years (SD ± 9.41) and 65.99 years (SD ± 10.45), respectively.
TABLE 1
| Number of newly diagnosed lung cancer patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |||||||
| Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | |
| Patients with new LC diagnosis (n) | 4,522 | 2,636 | 4,307 | 2,617 | 4,126 | 2,730 | 4,227 | 2,722 | 4,138 | 2,843 | 4,176 | 2,820 |
| Mean age at diagnosis (year) | 64.51 | 64.93 | 64.85 | 65.49 | 64.89 | 65.56 | 65.32 | 65.54 | 65.58 | 65.72 | 65.80 | 65.99 |
| Mean ± SD | ±9.85 | ±11.19 | ±9.83 | ±11.23 | ±9.95 | ±11.06 | ±9.68 | ±10.89 | ±9.48 | ±10.48 | ±9.41 | ±10.45 |
| Age group (n) | ||||||||||||
| 20–39 | 30 | 22 | 18 | 31 | 36 | 19 | 24 | 26 | 27 | 19 | 17 | 22 |
| 40–49 | 216 | 180 | 212 | 159 | 203 | 157 | 192 | 150 | 153 | 129 | 170 | 129 |
| 50–59 | 1,332 | 719 | 1,199 | 672 | 1,076 | 746 | 1,028 | 709 | 944 | 691 | 898 | 644 |
| 60–69 | 1,627 | 886 | 1,627 | 872 | 1,563 | 874 | 1,704 | 955 | 1,728 | 1,075 | 1,786 | 1,081 |
| 70–79 | 1,029 | 540 | 929 | 567 | 955 | 624 | 976 | 572 | 985 | 644 | 988 | 663 |
| 80–89 | 269 | 259 | 306 | 292 | 270 | 276 | 272 | 284 | 276 | 244 | 291 | 245 |
| 90≤ | 19 | 30 | 16 | 24 | 23 | 34 | 31 | 26 | 25 | 41 | 26 | 36 |
| Number of lung cancer patients died | ||||||||||||
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |||||||
| Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | |
| Number of Patients died (n) | 3,946 | 2,099 | 4,059 | 2,149 | 3,956 | 2,198 | 3,988 | 2,295 | 3,861 | 2,412 | 4,088 | 2,377 |
| Mean age at diagnosis (year) | 66.09 | 67.16 | 66.30 | 67.38 | 66.54 | 67.51 | 66.66 | 67.70 | 66.97 | 67.92 | 67.55 | 68.64 |
| Mean ±SD | ±9.83 | ±11.22 | ±9.97 | ±10.93 | ±9.66 | ±11.10 | ±9.79 | ±10.73 | ±9.49 | ±10.54 | ±9.37 | ±10.14 |
| Age group (n) | ||||||||||||
| 20–39 | <10 | <10 | 13 | <10 | 10 | <10 | 14 | <10 | 11 | <10 | <10 | <10 |
| 40–49 | 134 | 109 | 152 | 89 | 130 | 99 | 135 | 92 | 123 | 74 | 104 | 56 |
| 50–59 | 1,023 | 498 | 997 | 507 | 915 | 512 | 860 | 491 | 751 | 480 | 742 | 390 |
| 60–69 | 1,411 | 636 | 1,457 | 712 | 1,500 | 694 | 1,559 | 792 | 1,535 | 861 | 1,653 | 901 |
| 70–79 | 1,019 | 539 | 1,046 | 507 | 1,037 | 548 | 1,038 | 563 | 1,082 | 631 | 1,174 | 684 |
| 80–89 | 329 | 280 | 377 | 298 | 336 | 296 | 348 | 320 | 325 | 314 | 373 | 297 |
| 90≤ | 23 | 31 | 17 | 31 | 28 | 45 | 34 | 29 | 34 | 45 | 38 | 44 |
Crude incidence and mortality of lung cancer by sex and age between 2011 and 2016. SD: standard deviation.
Annual number of lung cancer related deaths were between 3,964 (2011) and 4,088 (2016) for males, and 2,099 to 2,377 for females. The mean age at the time of death also increased from 66.09 (SD ± 9.83) to 67.55 years (SD ± 9.37) at males between 2011 and 2016 and from 67.16 (SD ± 11.22) to 68.64 years (SD ± 10.14) among females during the whole study period.
Age Specific Incidence Rates
Age-specific incidence rates were higher in men than in women in most age cohorts (Figure 1). Incidence rates for men peaked at 375.0/100,000 PSYs, in the age group of 70–79 years in 2011, declining to 326.7 by 2016, corresponding to a mean annual change of −2.00% (95% CI: 0.16%–2.14%; p = 0.0639). Although the crude number of newly diagnosed lung cancer patients was highest in the 60–69 age group in men (1,627 in 2011 and 1,786 in 2016), the highest age-specific incidence rates were observed in the age group 70–79 years (Supplementary Table S1).
FIGURE 1
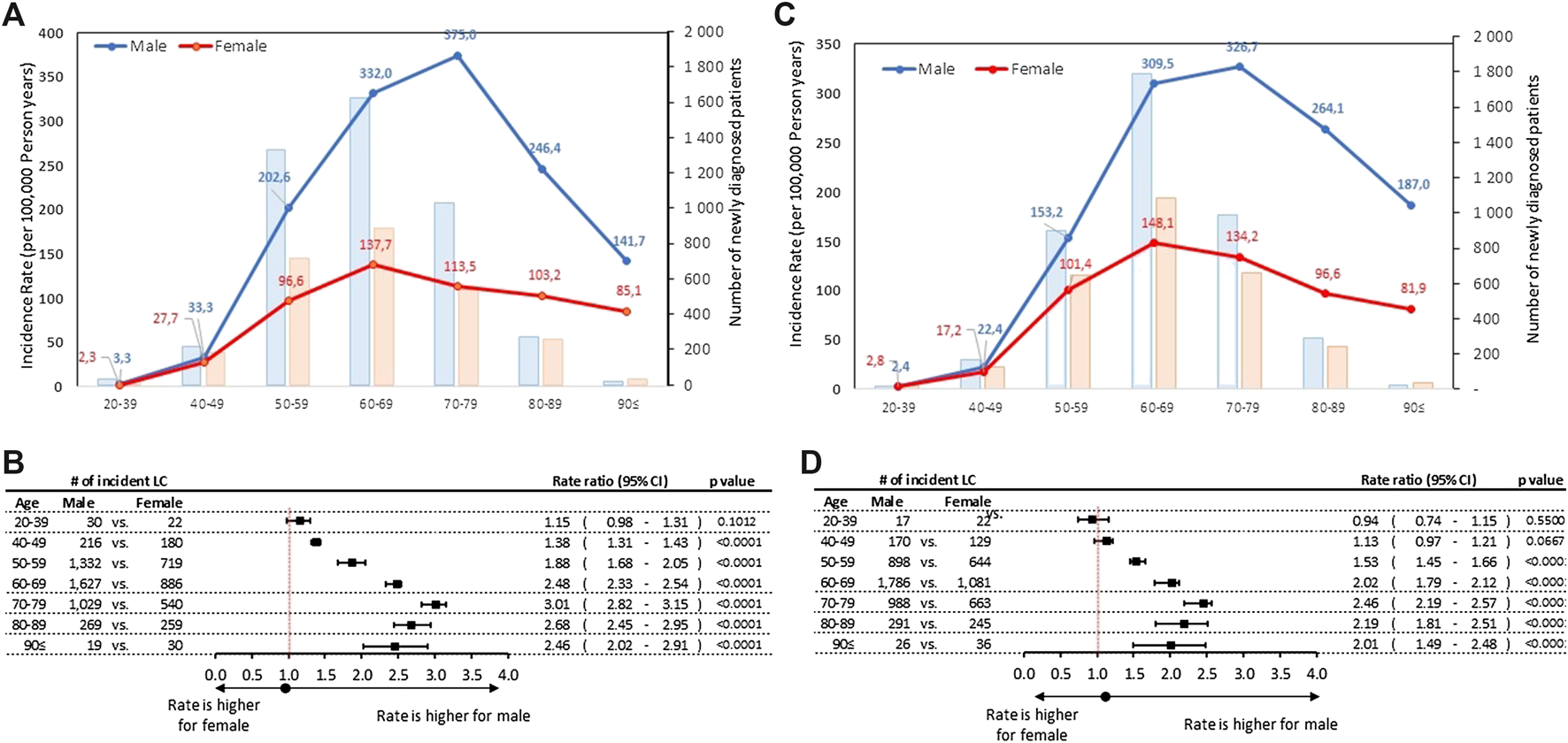
(A) Age-specific incidence rates and number of new lung cancer patients by sex in 2011 (B) Male-to-female incidence rate ratios by age cohorts in 2011 (C) Age-specific incidence rates and number of new lung cancer patients by sex in 2016 (D) Male-to-female incidence rate ratios by age cohorts in 2016. CI, confidence interval; LC, lung cancer.
Both incidence rates and crude numbers plateaued for women in the age group of 60–69 years (137.7 in 2011 and 148.1 in 2016), without any significant change (p = 0.1718) during the 6-years study period. Male-to-female rate ratios were higher among elderly patient: the highest statistically significant male-to-female rate ratio reached 3.01 (95% CI: 2.82–3.15; p < 0.0001) in the age group of 70–79 years in 2011, while the lowest was found to be 1.38 (95% CI:1.31-1.43; p < 0.0001) in the 40–49 age group in 2011. In the youngest age group (20–39 years) in 2016 the male to female ratio was 0.94, thus we found more incident female patients than male, but the rate ratio was not statistically significant.
Significant differences were found in the incidence rates during 6-years study period among male patients in the age groups of 40–49 and 50–59 years with a 11% (p = 0.0101) and 4% (p < 0.001) decrease, respectively (Figure 2A). We did not find any significant changes in incidence rates of lung cancer in 2016 vs. 2011 in other age groups in men. In women a significant increase in incidence rates was observed in the age groups of 60–69 years (RR: 1.03; 95% CI: 1.00–1.06; p = 0.0470) and 50–59 years (RR: 1.02; 95% CI: 1.01–1.05; p = 0.0277), while a 7% decrease was detected in the age group of 40–49 years (RR: 0.93; 95% CI: 0.88–0.97; p = 0.0192) (Figure 2B).
FIGURE 2
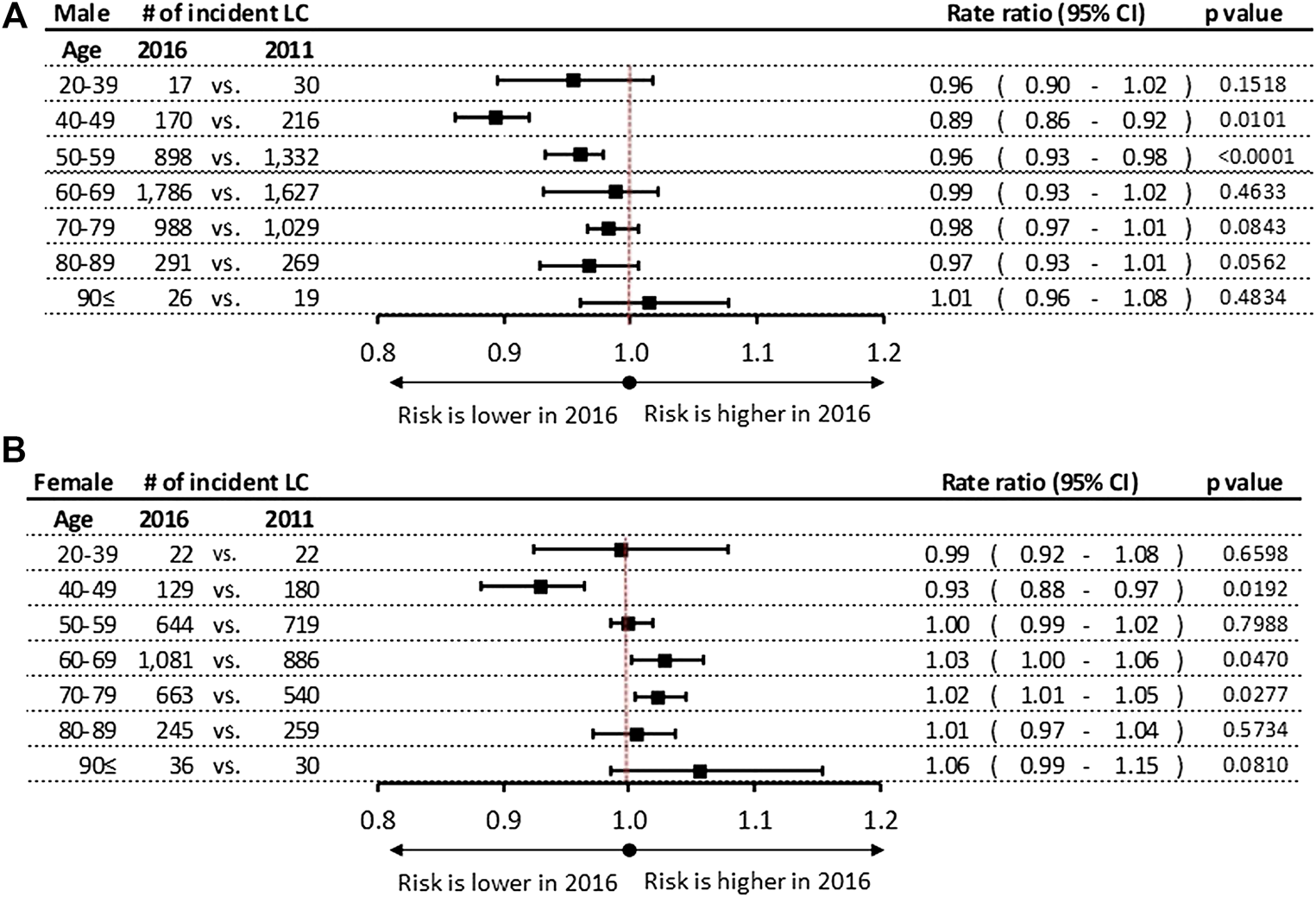
(A) Incidence rate ratios by age groups in men in 2016 vs. 2011. (B) Incidence rate ratios by age groups in women in 2016 vs. 2011. CI, confidence interval; LC, lung cancer.
Age Specific Mortality Rates
The rates of lung cancer mortality were also found to be higher among men, showing a sharp surge from the 50–59 age group and plateauing in the 70–79 years age-group with 368.9 and 385.0/100,000 PSYs in 2011 and 2016, respectively. (Figures 3A,C; Supplementary Table S2). However, the crude number of patients died peaked in 60–69 age group (1,411 and 636 in 2011 increased to 1,653 and 901 in 2016 at males and females, respectively). Male-to-female rate ratios were found to be highest with 3.38 in 2011 (95% CI: 3.18–3.67; p < 0.0001) declining to 2.82 (2.66–2.95; p < 0.0001) in 2016, respectively (Figures 3B,D).
FIGURE 3
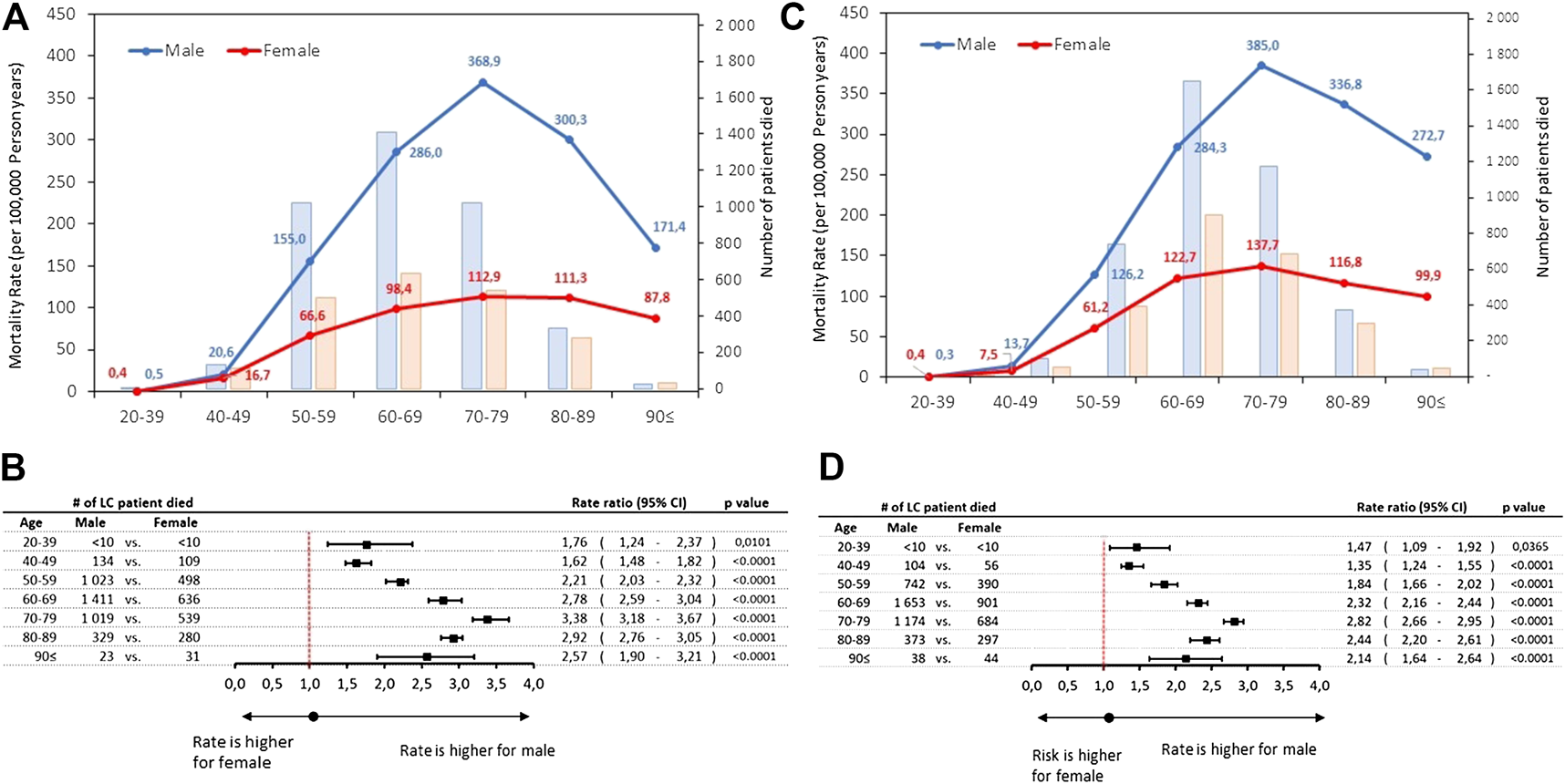
(A) Age-specific mortality rates and number of lung cancer death by sex in 2011 (B) Male-to-female mortality rate ratios by age cohorts in 2011 (C) Age-specific mortality rates and number of lung cancer death by sex in 2016 (D) Male-to-female mortality rate ratios by age cohorts in 2016. CI, confidence interval; LC, lung cancer.
A few statistically significant differences were found in the rates of mortality during the 6 years study period, showing varying trends by gender: we found a decrease among male patients in the age groups of 40–49 and 50–59 years in 2011 (RR: 0.89; 95% CI: 0.81–0.94, p < 0.0001 vs. 2016 (RR: 0.96;95% CI: 0.94-0.97, p < 0.0001) respectively. (Figure 4A). Inversely increase in mortality rates were found in the female age groups of 60–69, 70–79 and 80–89 years by 4% (95% CI: 1.02–1.06; p = 0.0189), 4% (95% CI: 1.02–1.08; p = 0.0101), and 3% (95% CI: 1.00–1.04; p = 0.0084), respectively (Figure 4B). In contrast, a decrease of 8% was found in female mortality rates in the age group of 40–49 years during the 6-years study period.
FIGURE 4
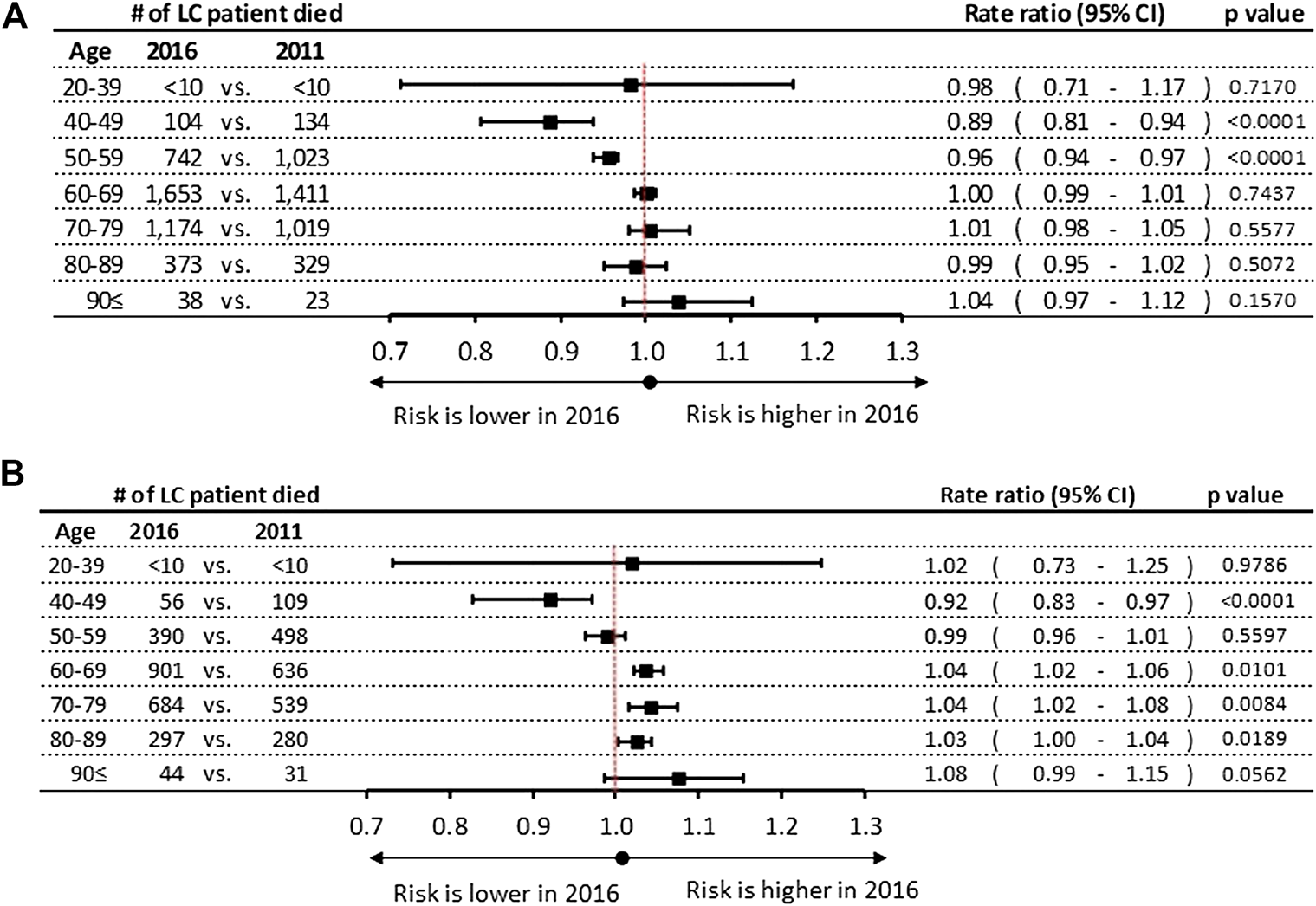
(A) Mortality rate ratios by age groups in 2016 vs. 2011 in men (B) Mortality rate ratios by age groups in 2016 vs. 2011 in women. CI, confidence interval; LC, lung cancer.
Discussion
This nationwide, retrospective, longitudinal study aimed to provide a comprehensive review of the age and gender specific incidence and mortality of lung cancer in Hungary, using a novel approach in data collection and study design based on the database of the National Health Insurance Fund of Hungary.
The key findings of this database research covering 6 years can be summarized as follows:
1. Age-specific incidence rates of lung cancer were highest in the 70–79 age cohort for men, while it peaked in the 60–69 age group for women.
2. While lung cancer incidence rates showed decreasing trends in most age cohorts for men, for females we found an increase in older age groups.
Differences in lung cancer epidemiology based on gender is a quite thoroughly researched topic, showing similar trends across the developed world, with incidence primarily influenced by prevalence of smoking [14, 15]. Numerous developed countries have reached the peak of their respective tobacco-related lung cancer disease burden, thus incidence and mortality rates started to decline, exhibiting major differences by gender. Historically smoking was always more prevalent among men, leading to higher rates of lung cancer incidence and mortality worldwide [16, 17]. Women took up smoking at a later period, mostly after the Second World War, and their rates of cessation have lagged those of men, leading to a much later peak in lung cancer incidence. In the United States age-standardized incidence rates for males have declined from a peak of 102 in 1984 to 69/100,000 PSYs by 2009, displaying a strong correlation with the decline in smoking prevalence since the 1980’s [18]. However, the trend is showing an opposite curve for females, with age-standardized rates reaching 51/100,000 PSYs, a major increase since 1984, from 39/100,000 PSYs [19, 20].
Similar trends were found in our recently published nationwide NHIF database study where incidence rates among men declined significantly from 115.7 to 101.6 per 100,000 PSYs during the 6-years study period, equivalent to a decrease of 2.35 percent [11]. This trend mirrors the decreasing smoking prevalence rates among Hungarian men, declining from 44% in 1994 to 31% in 2014 [21]. In the current analysis, the highest, 11% reduction of male lung cancer incidence was found in the age group of 40–49 years, although a less pronounced but still significant 4% change was detected in the age group of 50–59 years. In our study, the decrease in the incidence of lung cancer was not significant in older male cohorts (>60 years), which can probably be attributed to the shorter observation period and relatively low patient numbers. Nevertheless, the significant decrease in middle-aged male population may reflect the efficacy of anti-smoking initiatives in Hungary in the last decades [22]. On the other hand, we found increasing rates of lung cancer incidence in the whole female study population, with an annual increase of 2.37%. However, detailed age dependent analysis shows mixed results, the increase was significant in older age groups, while a decrease was detected in the age group of 40–49 years. These findings correspond to a study by Thomas et al. that compared the incidence of non-small cell lung cancer (NSCLC) in lung cancer patients younger and older than 40 years and found decreasing trends between 1975 and 2010 in both cohorts of males, however opposite trends in females lung cancer population, where younger patients had already decreasing trend on incidence, while older (>40) had still increase in incidence rate [23]. Akhtar-Danesh et al. also reported a decrease in the incidence of lung cancer among women in the age group of 50–59 years, and an increase over the age of 60 [6]
Differences in incidence of lung cancer among the genders could be explained by several factors, however, it can be concluded that the most important risk factor for the development of lung cancer is smoking in both genders [14, 24]. Smoking among women has significantly increased since the 1960s, resulting in a consequent increase in the risk of death in the female population [25]. The window of our study most probably covers a period, when we could capture the impact of changing smoking habits due to impact of Hungarian government’s anti-smoking campaign [22]. The European Health Interview Surveys reported smoking prevalence data from Hungary. In 2000, the smoking prevalence of males in the 18–34, 35–64, 65≤ age cohorts were 44.4%, 41.1%, and 13.7% respectively, while by 2 these rates decreased to 36.3% and 36.4% in 18–34 and 35–64 age groups, and slightly increased to 14.1% in the oldest one, showing a 18.2 and 11.2% decrease for the two younger cohorts. On the other hand, we could find a 11.7% decrease at females only in the 18–34 age group (29.0% onto 25.6% during 2000–2009 period), but plateauing trend was seen in the older female population (28.2%–28.8% and 3.4–7.0%) [26]. In their latest report describing smoking habits in Hungary Cselkó et al. reported that smoking prevalence have almost equalized among the genders: the proportion of male smokers showing a steady reduction (32–34%), with the prevalence of smoking stabilizing at 24–25% of the population [27]. This shifting prevalence of smoking is also in line with our findings showing a diminishing difference in the risk of lung cancer incidence and mortality between the genders in all age cohorts. This steady decrease in smoking prevalence at all male age cohorts during the 2000s years could contribute to the decrease of lung cancer incidence in the 2010s, while the peaking smoking prevalence in females could be explained by the increasing lung cancer incidence at most age groups of our study population. The link between smoking and LC is reinforced by a study involving diagnosed lung cancer patients from 2012, that looked at previous smoking status and found that the majority of patients had a history of smoking (79%), including 68% current and 32% former smokers. [28]. The increasing incidence of adenocarcinoma among non-smoking women may also play a role in the narrowing gap between males and females [29]. Other than smoking, several environmental risk factors (air pollution, exposure to asbestos, or radon) may also play a significant role in the changing lung cancer prevalence in both sexes. The impact of an environmental factor beyond smoking may play relevant role in changing prevalence of LC, especially if the size of impact is comparable with smoking habits, however a study examining air pollution trends in Hungary over the last 30 years found that most pollutants have decreased or at least stagnated, hence we believe smoking can be an explanatory factor [30]. Other than controlling exposure to pollutants, the introduction of a comprehensive and rigorously enforced anti-smoking laws of 2011 and 2013, the reduction in retail tobacco selling points, the new multi-media awareness campaign focusing on young generations and the foundation of a Methodological Support Centre for Smoking Cessation at the National Korányi Institute of Pulmonology may have further positive effects in the future by improving lung cancer trends in upcoming decades, in both sexes.
It is observed that the incidence of lung cancer shows strong correlation with age: rates usually start to increase from the age group of 45–49 years, with the highest rates observed in the 85–89 age group among men and in the 80–84 age group among women [31, 32]. In the United Kingdom Cancer Research database, we found similar result: male incidence peaked in 80 or above age cohort close to 600 per 100,000 PSYs, while the rate did not exceed the 100 per 100,000 PSYs till the age of 50 [5]. The peak of incidence was also found in the age group of 80–85 years in the United States as reported by Lung Cancer Statistics, while the highest rate among women was found in the 75–80 age group [7]. The Hungarian male age specific lung cancer incidence rate sharply increases from 40 to 49, exceeding 100 per 100.000 PSYs already by the 50 to 59 age cohorts, peaking in the 60 to 79 age groups, then sharply decreases at 80≤ age groups in both genders. This result is partly different from United Kingdom and United States trends where the peak of incidence was found in older age groups. The United Kingdom Cancer Research database [5] gives detailed tobacco smoking prevalence information, indicating lower smoking prevalence in the younger male and female age groups compared to Hungary but no relevant differences could be found in the older age groups. We could find similar differences in the age specific mortality rates. The earlier peak in the Hungarian lung cancer incidence could be explained by the higher smoking rates, however the lower incidence and mortality rates above the 80 age cohort could be explained by the higher cardiovascular risk of Hungarian population [33], acting as a competing morbidity, resulting in earlier mortality especially among regular heavy smokers [34].
To conclude, our study is the first nationwide investigation describing the age and sex specific incidence and mortality rates of lung cancer in Hungary, showing declining trends in most age groups of males, and an increasing incidence in the older female population in parallel with international trends. The highest incidence rates were found at 70–79 age cohort in males and at 60–69 age cohorts in females, earlier compared to western countries, probably due to the higher competing risk of cardiovascular diseases in elderly people.
There are certain strengths and limitations of our study. The coverage of the whole population in the NHIF database to identify lung cancer patients, the carefully cleaned data, the 6-years-long follow-up period, all provide a solid foundation for drawing conclusions from our analysis. Nevertheless, NHIF database does not contain any data on the staging or the ECOG status of patients, and no laboratory test results were available. Besides, we were not able to examine the competing cause of death in the lung cancer population, and also have no information on the share of active/passive smokers in the population or the rate of non-smoking dependent lung cancer.
Our study emphasizes the needs of putting more focus on the increasing female lung cancer incidence and reinforces the need of more detailed researches to understand more the underlying causes of these negative trends as well as to put more effort on the screening of younger smoker population.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Approval was obtained from the Central Ethical Committee of Hungary (10338–5/2019/EKU C).
Author contributions
LT, KB, GO, VM, LU, and NB contributed in finalizing the clinical aspects of the manuscript. AV, ZP, and ZK provided essential project management support, while GR and ZA-T helped with collecting and understanding the source data used for analysis. KH, BN ,and ZV helped with the data interpretation and analysis. GG, SV, and JM provided clinical insights during the preparation of the manuscript, which helped us validate our findings and conclusions.
Funding
The programme is financed by MSD Pharma Hungary Ltd. JM was supported by the Hungarian Brain Research Program (grant 2017-1.2.1-NKP-2017-00002), and the Hungarian NRDI Office (grant K-129065).
Conflict of interest
ZK, AV, and ZN-E, and AD are employees of MSD Pharma Hungary Ltd. KH is a research fellow at Eötvös Loránd University. BN and ZV are employees of Eötvös Loránd University where their contribution to this project was financially compensated. KB and GO are employees of National Korányi Institute of Pulmonology and have received speaker honorarium from MSD Hungary. GG is employee of Oncology Center of Törökbálint and has received speaker honorarium from MSD Hungary, LT and VM are employees of Semmelweis University and they declare have no conflict of interest, LU is employee of MG and he declares have no conflict of interest, NB is employee of University of Debrecen and she declares have no conflict of interest, VS is employee of University of Pécs and she declares have no conflict of interest. GR and ZA-T are employees of RxTarget Ltd. where their contribution to this project was financially compensated. ZB is employees of Synthesia Ltd. And her contribution to this project was financially compensated.
The authors declare that this study received funding from MSD Pharma Hungary Ltd. The funder had the following involvement with the study: study design, data collection and analysis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2021.598862/full#supplementary-material
References
1.
FerlayJ.SoerjomataramI.DikshitR. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Canc.136 (5), E359–E386. 10.1002/ijc.29210
2.
AlbergA. J.SametJ. M. (2003). Epidemiology of lung cancer*. Chest123 (1), 21S–49S. ISSN 0012-3692.
3.
Cancer Research UK (2019). Data are from Cancer Research UK portal, referenced per instruction on the homepage. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer#heading-Three (Accessed September 24, 2019).
4.
Patricia deG.MundenR. F. (2012). Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin.50 (5), 863–876. 10.1016/j.rcl.2012.06.006
5.
Cancer Research UK database (2019). Data were provided by the Cancer Research UK database. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#ref (Accessed October 6, 2019).
6.
Akhtar-DaneshN.FinleyC. (2015). Temporal trends in the incidence and relative survival of non-small cell lung cancer in Canada: a population-based study. Lung Canc.90 (1), 8–14. 10.1016/j.lungcan.2015.07.004
7.
TorreL. A.SiegelR. L.JemalA. (2016). Lung cancer statistics. Adv. Exp. Med. Biol.893, 1–19. 10.1007/978-3-319-24223-1_1
8.
MalvezziM.BertuccioP.RossoT. (2015). European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women?Ann. Oncol.26 (4), 779–786. 10.1093/annonc/mdv001
9.
PaggiM. G.VonaR.AbbruzzeseC.MalorniW. (2010). Gender-related disparities in non-small cell lung cancer. Cancer Lett.298 (1), 1–8. 10.1016/j.canlet.2010.08.009
10.
OberliL. S.ValeriF.KorolD. (2016). 31 years of lung cancer in the canton of Zurich, Switzerland: incidence trends by sex, histology and laterality. Swiss Med. Wkly.146, w14327. 10.4414/smw.2016.14327eCollection 2016
11.
BogosK.KissZ.GálffyG.TamásiL.OstorosG.MüllerV.et al (2019). Revising incidence and mortality of lung cancer in Central Europe: an epidemiology review from Hungary. Front. Oncol.9, 1051. 10.3389/fonc.2019.01051
12.
StatinfoK. S. H. (2019). http://statinfo.ksh.hu/Statinfo/index.jsp (Accessed November 24, 2019).
13.
European Commission (2013). Revision of the European standard population—report of Eurostat’s task force. Luxembourg: publications office of the European union. Available at: https://ec.europa.eu/eurostat/documents/3859598/5926869/KS-RA-13-028-EN.PDF/e713fa79-1add-44e8-b23d-5e8fa09b3f8f (Accessed March 9, 2019).
14.
JemalA.ThunM. J.RiesL. A. (2008). Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J. Natl. Canc. Inst.100, 1672–1694. 10.1093/jnci/djn389
15.
McGuireS. (2013). World cancer report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer, WHO Press, 2015. Adv. Nutr.7, 418–419. 10.3945/an.116.012211
16.
YouldenD. R.CrambS. M.BaadeP. D. (2008). The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J. Thorac. Oncol.3, 819–831. 10.1097/JTO.0b013e31818020eb
17.
SiegelR. L.MillerK. D.JemalA. (2018). Cancer statistics. CA Canc. J. Clin.68, 7–30. 10.3322/caac.21442
18.
HakulinenT.EngholmG.GislumM. (2010). Trends in the survival of patients diagnosed with cancers in the respiratory system in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol.49 (5), 608–623. 10.3109/02841860903575281
19.
FuJ. B.KauT. Y.SeversonR. K. (2005). Lung cancer in women: analysis of the national surveillance, epidemiology, and end results database. Chest127 (3), 768–777. 10.1378/chest.127.3.768
20.
CaroleA. R.AoifeM. (2013). McErlean. Epidemiology of lung cancer. Semin. Intervent. Radiol.30, 93–98. 10.1055/s-0033-1342949
21.
TomborI.PaksiB.UrbánB. (2011). Epidemiology of smoking in the Hungarian population, based on national representative data. Clin. Exp. Med. J.5 (1), 27–37. 10.1556/CEMEd.4.2010.28817
22.
JoóT.VokóZ.BodrogiJ. (2017). Anti-smoking actions between 2010–2014. IME.XVI (10), 11–17.
23.
ThomasA.ChenY.YuT. (2015). Trends and Characteristics of young non-small cell lung cancer patients in the United States. Front. Oncol.5, 113. 10.3389/fonc.2015.00113eCollection 2015
24.
Dela CruzC. S.TanoueL. T.MatthayR. A.Lung cancer: epidemiology, etiology, and prevention. Clin. Chest Med.32 (4), 605–644. 10.1016/j.ccm.2011.09.001
25.
RiveraM. P. (2013). Lung cancer in women: differences in epidemiology, biology, histology, and treatment outcomes. Semin. Respir. Crit. Care Med.34 (6), 792–801. 10.1055/s-0033-1358550
26.
KSH (2009). Európai Lakossági Egészségfelmérés Statisztikai Tükör IV évfolyam, 50. Szám 2010. http://www.ksh.hu/docs/hun/xftp/gyor/jel/jel310021.pdf (Accessed November 28, 2019), 1–7.
27.
CselkóZ.KovácsG. (2013). Smoking habits in Hungary: analysis based on surveys of the past decade. Hungarian. Orv Hetil.154 (37), 1454–1468. 10.1556/OH.2013.29694
28.
KovácsG.BarsaiA.SzilasiM. (2012). A dohányzás, mint a tüdõrákos betegek túlélésének prognosztikai tényezõje [Smoking: a prognostic factor of lung cancer survival]. Magy. Onkol.56 (3), 187–191.
29.
TaioliE.WynderE. L. (1994). Re: Endocrine factors and adenocarcinoma of the lung in women. J. Natl. Canc. Inst.86 (11), 869–870. 10.1093/jnci/86.11.869
30.
European Environment Agency (2014). Air pollution fact sheet, Hungary. Available at: https://www.eea.europa.eu/themes/air/air-pollution-country-fact-sheets-2014/hungary-air-pollutant-emissions-country-factsheet+&cd=1&hl=hu&ct=clnk&gl=qa (Accessed September 20, 2020).
31.
AareleidT.ZimmermannM. L.BaburinA. (2017). Divergent trends in lung cancer incidence by gender, age and histological type in Estonia: a nationwide population-based study. BMC Canc.17 (1), 596. 10.1186/s12885-017-3605-x
32.
ThakkarJ. P.McCarthyB. J.VillanoJ. L. (2014). Age-specific cancer incidence rates increase through the oldest age groups. Am. J. Med. Sci.348 (1), 65–70. 10.1097/MAJ.0000000000000281
33.
TimmisA.TownsendN.GaleC. (2018). European society of cardiology: cardiovascular disease statistics 2017. Eur. Heart J.39 (7), 508–579. 10.1093/eurheartj/ehx628
34.
TaghizadehN.VonkJ. M.BoezenH. M. (2016). Lifetime smoking history and cause-specific mortality in a cohort study with 43 years of follow-up. PLoS One.11 (4), e0153310. 10.1371/journal.pone.0153310
Summary
Keywords
epidemiology, lung cancer, incidence, mortality, age and gender
Citation
Tamási L, Horváth K, Kiss Z, Bogos K, Ostoros G, Müller V, Urbán L, Bittner N, Sárosi V, Vastag A, Polányi Z, Nagy-Erdei Z, Daniel A, Nagy B, Rokszin G, Abonyi-Tóth Z, Moldvay J, Vokó Z and Gálffy G (2021) Age and Gender Specific Lung Cancer Incidence and Mortality in Hungary: Trends from 2011 Through 2016. Pathol. Oncol. Res. 27:598862. doi: 10.3389/pore.2021.598862
Received
25 August 2021
Accepted
28 September 2021
Published
30 April 2021
Volume
27 - 2021
Edited by
Andrea Ladányi, National Institute of Oncology (NIO), Hungary
Reviewed by
Istvan Bodi, King’s College London, United Kingdom
Edit Bardi, St.Anna Kinderspital, Austria
Updates
Copyright
© 2021 Tamási, Horváth, Kiss, Bogos, Ostoros, Müller, Urbán, Bittner, Sárosi, Vastag, Polányi, Nagy-Erdei, Daniel, Nagy, Rokszin, Abonyi-Tóth, Moldvay, Vokó and Gálffy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krisztián Horváth, horvath.krisztian@tatk.elte.hu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.