Abstract
Aims: β-catenin is a critical regulating factor of the Wnt pathway, which is closely linked to tumorigenesis, tumor growth, metastasis, and tumor immunity. Our study focused on exploring the relationship between β-catenin and clinicopathological features, prognosis, as well as infiltrating immune cells and immune scores, so as to illustrate its clinical significance in NSCLC.
Materials and Methods: The β-catenin mRNA (CTNNB1) and protein expression data were downloaded from the UALCAN and the UCSC Xena website, respectively. All tumor-immune infiltrating cells’ data were downloaded from the TIMER platform and immune scores were downloaded from ESTIMATE website. The expression of β-catenin protein in our cohort was measured by immunohistochemistry.
Results: β-catenin mRNA level was higher in lung adenocarcinoma (LUAD) compared to normal tissues (p < 0.001) and was related to overall survival (OS) (p < 0.001) and post-progression survival (PPS) (both p = 0.049) in LUAD. Aberrant β-catenin protein expression was higher in male and lung squamous cell carcinoma (LUSC) patients (both p = 0.001). Also, it was considered to be a prognosis factor independently (p = 0.034). In addition, β-catenin protein was negatively correlated with CD8+T cells (r = −0.128, p = 0.008), neutrophils (r = −0.198, p < 0.001), immune score (r = −0.109, p = 0.024), stromal score (r = −0.097, p = 0.045), and ESTIMATE score (r = −0.113, p = 0.020).
Conclusions: Aberrant β-catenin protein expression was evidently higher in NSCLC and might serve as a biomarker for poor prognosis. Most importantly, β-catenin protein might play an important part in tumor immunity and the tumor microenvironment by inhibiting the infiltration of CD8+ T cells and neutrophils.
Introduction
Lung cancer, which is one of the most principal malignant cancers with increasing incidence rates year by year, often occurs in bronchial mucosal epithelium and alveoli [1,2]. It is divided into SCLC (small-cell lung cancer) and NSCLC (non-small cell lung cancer), and then further divided into squamous cell carcinoma, adenocarcinoma, and large-cell lung cancer [3]. Most patients are asymptomatic, and the tumor may progress when the patient has respiratory tract or tumor-related symptoms [4]. Although chemoradiotherapy and targeted therapy (such as EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor) have brought unprecedented survival benefits to some patients, all these treatments are prone to drug resistance and have some side effects [5,6]. In recent years, immuno-checkpoint inhibitor (ICI), represented by monoclonal antibodies against programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1), can cause long-term remission and even cure 20–30% of patients. It is a further sign of revolutionary progress in the field of lung cancer treatment, but not all patients can benefit from immunotherapy, and the overall effective rate is low [7,8]. The occurrence and development of NSCLC is an extremely complex process involving multiple steps, multiple factors, and multiple genes. Consequently, it is urgent to study the exact pathogenesis of non-small cell lung cancer and find accurate biomarkers for predicting prognosis and searching for new clues for therapeutic targets.
β-catenin is a multifunctional protein, which was first discovered as a mammalian cell adhesion complex in 1990. It can interact with cadherin, participate in cell adhesion, and form cytoskeletons [9,10]. The imbalance of β-catenin structure and signal properties often leads to disease and uncontrolled growth of cancer [11]. Also, it is a critical regulating factor of the Wnt pathway. When the classical Wnt pathway is activated, the cytoplasmic β-catenin is transferred to the nucleus and then interacts with some transcription factors (such as LEF, TCF, and BCL-9) to further activate target genes of the pathway [12,13]. Abnormal activation of the Wnt/β-catenin pathway exists widely in different types of malignant tumors, such as lung cancer, pancreatic cancer, liver cancer, and colorectal cancer, and is closely associated with tumorigenesis, tumor growth, metastasis, and tumor immunity [14–16].
Tumor microenvironment, as the living environment of tumor cells, is composed of various cytokines/chemokines, immune cells, tumor-related fibroblasts, inflammatory cells, and microvessels and has a significant impact on the treatment response and overall prognosis of tumor patients [17,18]. Reshaping the immune response of the immune microenvironment is helpful to improve the prognosis of patients. Crosstalk between Wnt/β-catenin signaling and immune cells has both positive and negative effects on tumor progression. It helps to maintain and renew the leucocytes, but also promotes immune tolerance and limits anti-tumor response [19]. The relevance between Wnt/β-catenin signaling and immune microenvironment is complicated in NSCLC. Therefore, this study will explore the potential correlation between β-catenin protein and clinicopathological/prognostic features, as well as its association with tumor-infiltrating immune cells and immune score.
Materials and Methods
Data Collection
The expression of CTNNB1 (β-catenin mRNA) and β-catenin protein in The Cancer Genome Atlas (TCGA) database was downloaded from the UALCAN (http://ualcan.path.uab.edu/) and the UCSC Xena website (http://xena.ucsc.edu/), respectively. All tumor-immune infiltrating cells data (including macrophages, CD4+T-cells, B-cells, neutrophils CD8+T-cells, and dendritic cells) were obtained from the Tumor Immune Estimation Resource (TIMER) platform (https://cistrome.shinyapps.io/timer/). The immune score, stromal score, and estimate score were downloaded from ESTIMATE (https://bioinformatics.mdanderson.org/estimate/). K-M plotter (http://kmplot.com/analysis/index.php?p=service#) was applied to analyze the potential prognostic value of CTNNB1 in NSCLC, which was divided into low and high groups according to the median level [20].
Patients Cohorts
We collected 273 cases of NSCLC postoperative samples from Central South University, The Second Xiangya Hospital. There were 133 cases of LUSC, 140 of LUAD, and 106 non-cancerous lung tissues from 2003 to 2013, including 70 female and 203 male patients with a median age of 56 years. Of the cases, 132 were at clinical stage I and II and 141 cases were at stage III. 154 cases had lymph node metastasis and 119 had no lymph node metastasis. At the last follow-up, 164 patients had survived and 109 patients had died. All patients had complete follow-up and clinical data. None of them had received treatment before surgery. Each case had definite clinical stage and pathological diagnosis on the basis of the Eighth Edition Lung Cancer Stage Classification and the WHO histological classification, respectively [21].
IHC and Scores
The β-catenin protein with a dilution of 1:100 (Rabbit mAb #8480, Cell Signaling Technology) was examined according to the protocols as described before [22]. In short, the slides were dewaxed and rehydrated, repaired at high temperature with sodium citrate for 10 min, blocked with 3% hydrogen peroxide, and finally incubated with primary antibody in a wet box at 4°C overnight. Each experiment included negative and positive controls.
The Immunohistochemical score was independently performed under 200x light microscopy by YZ and SF who were blinded to patients’ clinical information. The score evaluation was as follows [23]: normal expression was defined as more than 70% of the cell membrane having positive staining for β-catenin and less than 60% of the cytoplasm or the nucleus showing positive staining, the others were defined as aberrant expression. All discordances were resolved through discussion.
Statistical Analysis
SPSS 24.0 was applied to all statistical analyses. The correlation between β-catenin protein level and clinicopathological factors of NSCLC was examined by chi-square test. Besides, the relationship among β-catenin protein, tumor-immune infiltrating cells, and immune score was examined by Spearman’s correlation test. Survival situation was tested by Kaplan-Meier analysis and Log-rank test. Cox hazards model was applied to evaluate the independent prognostic factors of NSCLC. p < 0.05 (two-sided) was considered to be statistically significant.
Results
Correlation Between Aberrant Expression of β-catenin and Clinicopathological Variables in NSCLC
In UALCAN database, CTNNB1 mRNA expression was up-regulated in LUAD (Figure 1, p < 0.001) but not in LUSC (p > 0.05). We also detected the prognostic value of CTNNB1. Low CTNNB1 mRNA expression was related to better OS (overall survival) and PPS (post progression survival) in LUAD (Figure 1, both p < 0.05), but had no correlation in LUSC (both p > 0.05).
FIGURE 1
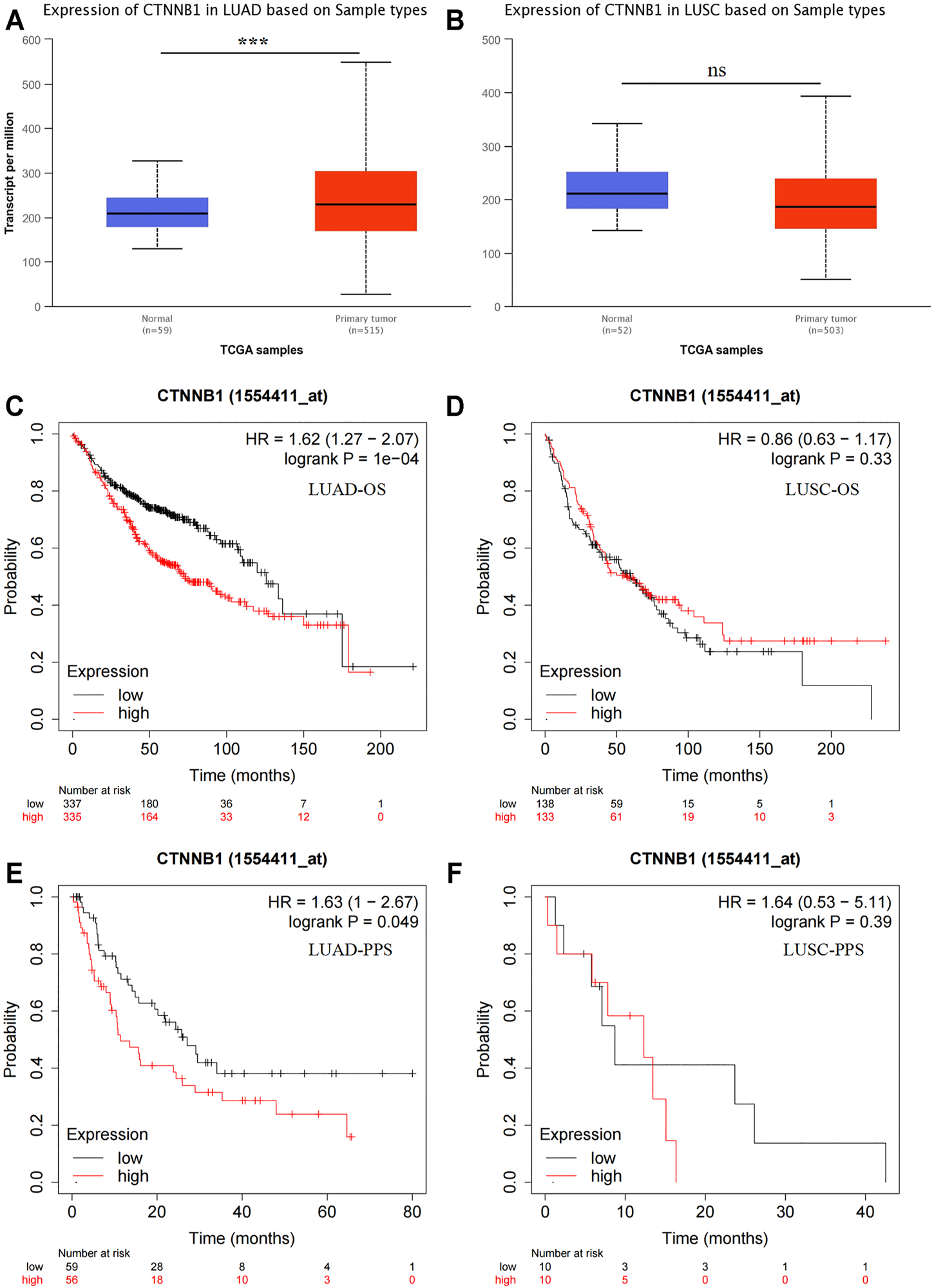
Bioinformatics analysis of β-catenin mRNA expression (CTNNB1). Expression of CTNNB1 in LUAD (A) and LUSC (B). OS analysis of CTNNB1 in LUAD (C) and LUSC (D). PPS analysis of CTNNB1 in LUAD (E) and LUSC (F). OS, overall survival; PPS, post-progression survival.
In order to explore β-catenin protein expression in our patient cohort, we used IHC to detect the subcellular localization and expression of β-catenin protein in NSCLC and Non-CLT (non-cancerous control lung tissue). β-catenin protein was chiefly distributed in the cell membrane/cytoplasm, as well as in the nucleus (Figure 2). Aberrant β-catenin protein expression was 78.8% (215/273) in NSCLC but was 48.1% (51/106) in non-cancerous control lung tissues (Figure 3). Specifically, β-catenin protein aberrant expression was 70.7% (99/140) in LUAD and 87.2% (116/133) in LUSC tissues. We further analyzed the link between β-catenin protein and clinicopathological variables (including pathological grade, clinical stage, age, gender, and histological type) by chi-square test. Results in Table 1 showed that aberrant β-catenin protein expression rate was increased in male and LUSC patients (both p = 0.001) compared to female and LUAD patients. It is worth noting that aberrant β-catenin protein expression rate was higher in clinical stage III patients compared to stage I patients, but the statistical difference was weak (p = 0.078). No obvious statistical difference was found in other clinicopathological variables (p > 0.05).
FIGURE 2
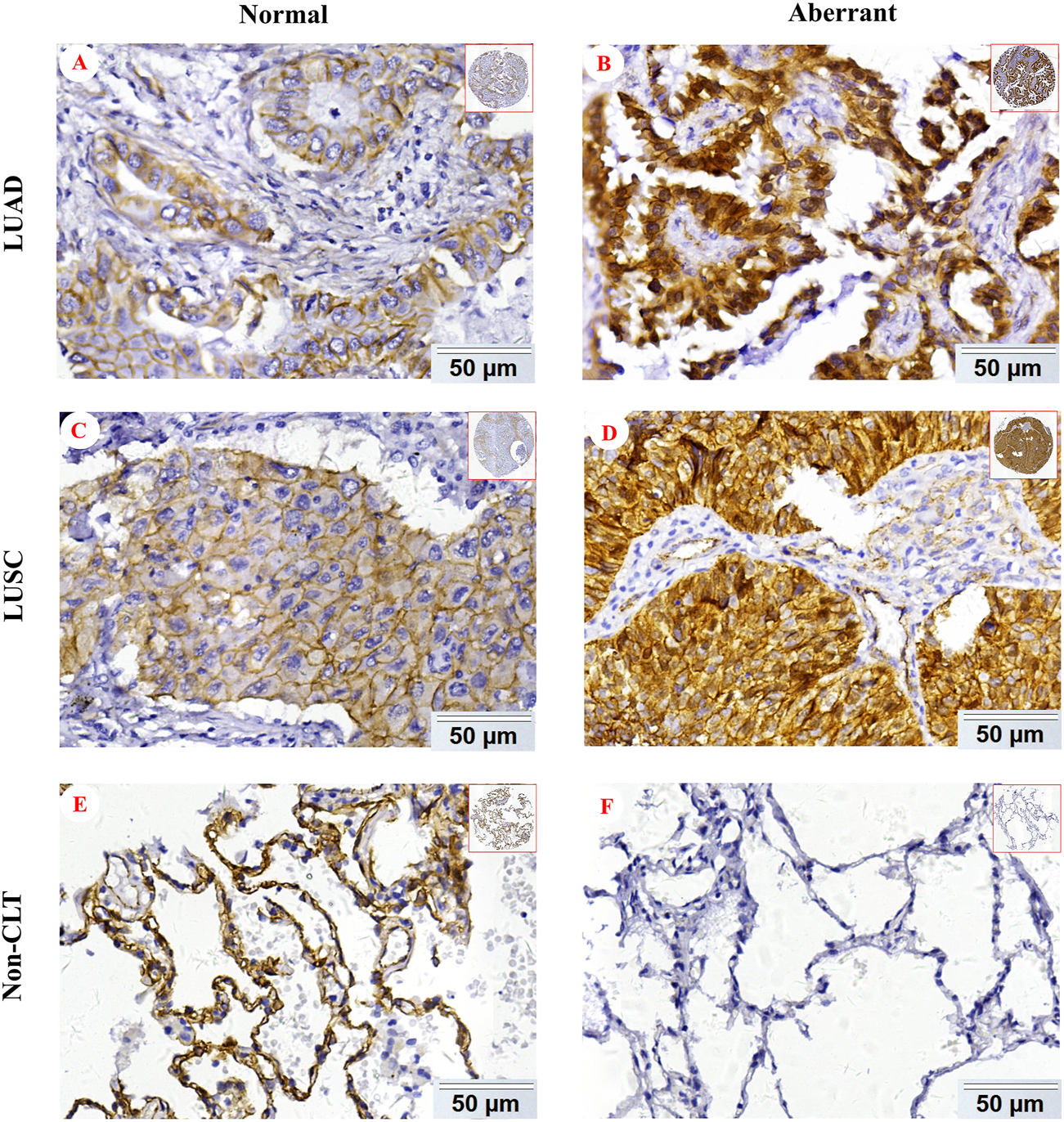
Representative IHC images of β-catenin protein in NSCLC. Normal β-catenin protein expression in LUAD (A), LUSC (C), and Non-CLT (E). Aberrant β-catenin protein expression in LUAD (B), LUSC (D), and Non-CLT (F). Non-CLT: non-cancerous control lung tissue.
FIGURE 3
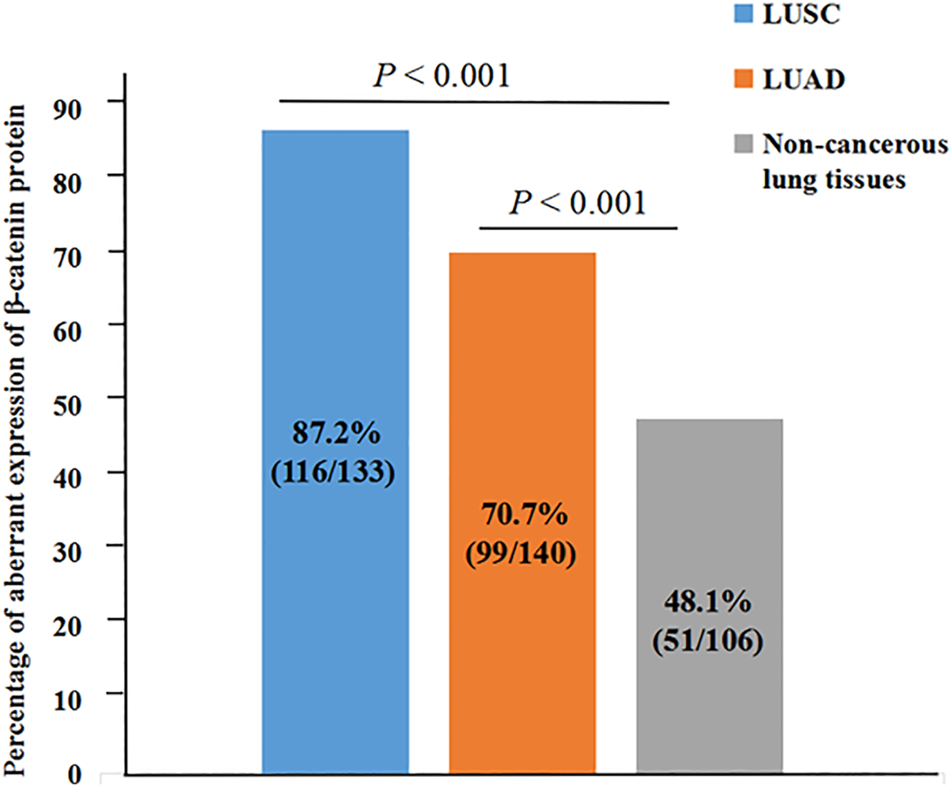
Aberrant expression rate of β-catenin protein in NSCLC and non-cancerous lung tissues. Aberrant β-catenin protein expression rate was increased in LUSC and LUAD (both p < 0.001).
TABLE 1
| Variables | β-catenin | ||
|---|---|---|---|
| Normal (%) | Aberrant (%) | p | |
| Age (years) | |||
| <60 (n = 177) | 37 (20.9%) | 140 (79.1%) | 0.851 |
| ≥60 (n = 96) | 21 (21.9%) | 75 (78.1%) | |
| Gender | |||
| Female (n = 70) | 25 (35.7%) | 45 (64.3%) | 0.001* |
| Male (n = 203) | 33 (16.3%) | 170 (83.7%) | |
| Histological type | |||
| LUAD (n = 140) | 41 (29.3%) | 99 (70.7%) | 0.001* |
| LUSC (n = 133) | 17 (12.8%) | 116 (87.2%) | |
| Pathological grade | |||
| Well/moderate (n = 128) | 31 (24.2%) | 97 (75.8%) | 0.259 |
| Poor (n = 145) | 27 (18.6%) | 118 (81.4%) | |
| Clinical stage | |||
| Stage I and II (n = 132) | 34 (25.8%) | 98 (74.2%) | 0.078 |
| Stage III (n = 141) | 24 (17.0%) | 117 (83.0%) | |
| LNM status | |||
| LNM (n = 154) | 29 (18.8%) | 125 (81.2%) | 0.267 |
| No LNM (n = 119) | 29 (24.4%) | 90 (75.6%) | |
Association between aberrant expression of β-catenin protein and clinicopathological features of NSCLC (n = 273).
*p < 0.05.
LUAD, lung adenocarcinoma; LNM, lymph node metastasis; LUSC, lung squamous cell carcinoma.
Effects of Aberrant β-Catenin Protein Expression on Prognosis of NSCLC Patients
In this study, survival status of NSCLC patients with different clinicopathological variables was analyzed through Kaplan-Meier survival analysis. For patients with NSCLC, the overall survival of patients with aberrant β-catenin protein expression was remarkably shorter than that of patients with normal protein expression (p = 0.018, Figure 4A). In addition, shorter survival time was identified in cases with LNM (lymph node metastasis; p < 0.001, Figure 4B), poor differentiation (p = 0.002, Figure 4C), and clinical stage III (p < 0.001, Figure 4D). We also analyzed the prognostic value of β-catenin protein in LUAD and LUSC independently (Supplementary Figure S1). We found that β-catenin protein had prognostic value in LUAD, but not in LUSC.
FIGURE 4
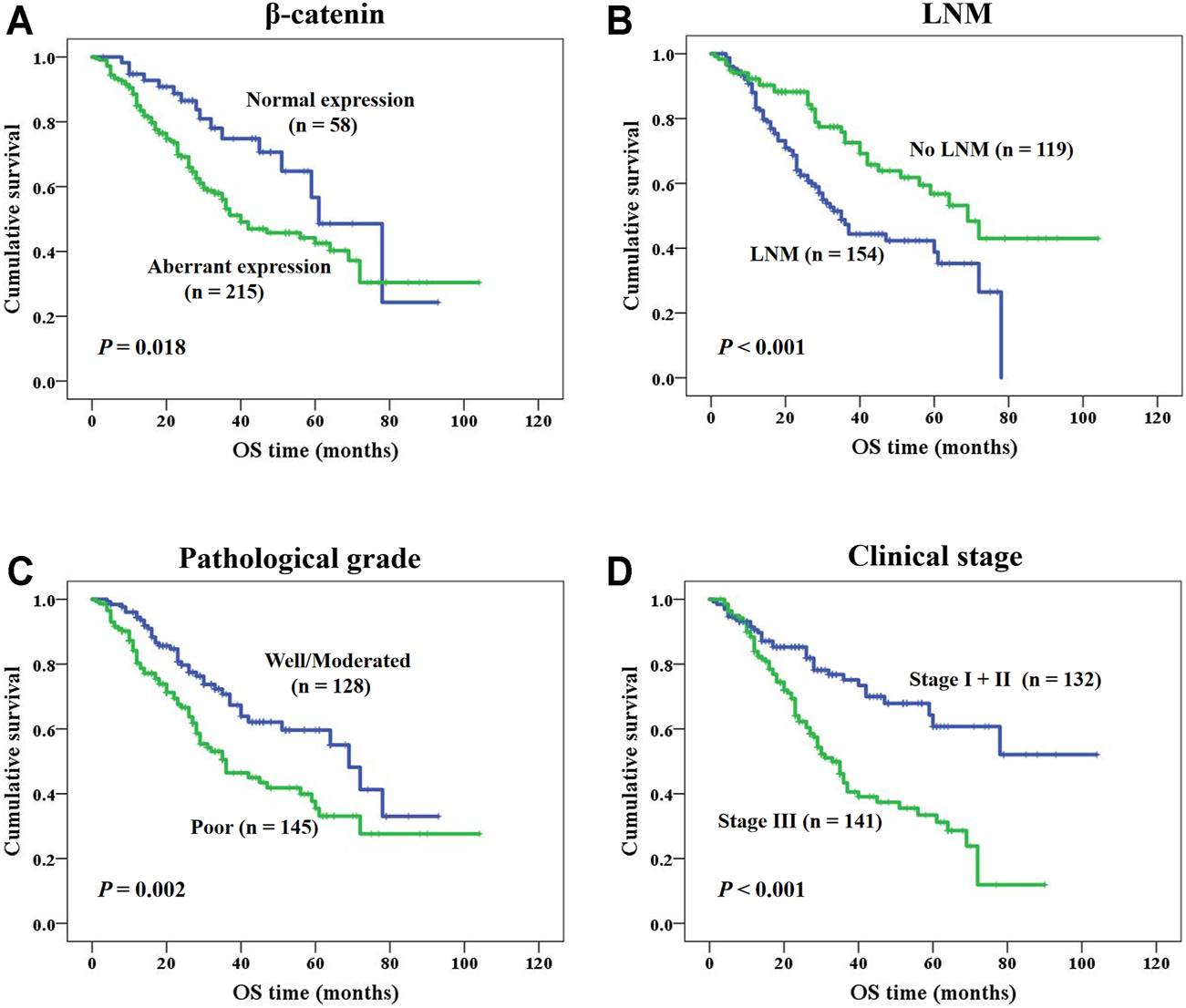
K-M survival analysis of NSCLC patients. (A) NSCLC patients with aberrant β-catenin protein expression had a shorter OS (p = 0.018). (B) NSCLC patients with LNM had a shorter OS (p < 0.001). (C) Patients with poor differentiated NSCLC had a shorter OS (p = 0.002). (D) NSCLC patients with clinical stage III had a shorter OS (p < 0.001).
Moreover, we also studied whether β-catenin protein expression status could be utilized as a prognostic factor for NSCLC patients independently. As shown in Table 2, we identified aberrant expression of β-catenin protein as a poor prognostic factor independently (p = 0.034), along with clinical stage III (p = 0.003), poor differentiation (p = 0.015), and with LNM (p = 0.019).
TABLE 2
| Parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Average survival time (SE) | 95% CI | p | Exp (B) | 95.0% CI | p | |
| β-catenin | ||||||
| Aberrant expression | 53.981 (3.506) | (47.110, 60.852) | 0.018* | 0.552 | (0.319, 0.956) | 0.034* |
| Normal expression | 61.862 (5.423) | (51.233, 72.491) | ||||
| Clinical stage | ||||||
| Stage I and II | 72.615 (4.644) | (63.513, 81.717) | 0.000* | 0.521 | (0.336, 0.806) | 0.003* |
| Stage III | 41.670 (2.947) | (35.894, 47.447) | ||||
| LNM status | ||||||
| LNM | 43.467 (2.698) | (38.178, 48.755) | 0.000* | 1.681 | (1.089, 2.596) | 0.019* |
| No LNM | 67.481 (4.643) | (58.382, 76.581) | ||||
| Pathological grade | ||||||
| Well and moderate | 60.332 (3.826) | (52.833, 67.831) | 0.002* | 0.614 | (0.414, 0.910) | 0.015* |
| Poor | 50.220 (4.116) | (42.153, 58.288) | ||||
| Histological type | ||||||
| LUAD | 50.983 (3.365) | (44.343, 57.534) | 0.798 | 1.382 | (0.919, 2.078) | 0.120 |
| LUSC | 61.293 (4.685) | (52.109, 70.477) | ||||
| Gender | ||||||
| Female | 60.240 (4.789) | (50.854, 69.625) | 0.077 | 0.672 | (0.417, 1.085) | 0.104 |
| Male | 53.347 (3.748) | (46.001, 60.692) | ||||
| Age | ||||||
| <60 | 58.663 (4.008) | (50.806, 66.519) | 0.107 | 0.816 | (0.552, 1.206) | 0.307 |
| ≥60 | 48.535 (4.352) | (40.006, 57.064) | ||||
Univariate and multivariate analysis for OS in NSCLC patients.
*p < 0.05.
OS, overall survival; CI, confidence interval; SE, standard error; Exp(β), odds ratio; LUAD, lung adenocarcinoma; LNM, lymph node metastasis; LUSC, lung squamous cell carcinoma.
Correlation Analysis Between β-Catenin Protein Expression and Immune Cell Infiltration Level and Immune Score
Firstly, we explored the relationship between β-catenin protein and tumor-immune infiltrating cells, including CD4+ T cells, neutrophils, CD8+ T cells, macrophages, dendritic cells, and B cells. Expression data of β-catenin protein were downloaded from the UCSC Xena website, whose expression level was detected by reverse phase protein array (RPPA). Tumor-immune infiltrating cell data were obtained from the TIMER platform (Tumor Immune Estimation Resource). As shown in Figure 5, we found that infiltrating levels of CD8+ T cells (p = 0.008, r = −0.128) and neutrophils (p < 0.001, r = −0.198) in the UCSC had significant negative correlation with β-catenin protein expression level. However, there were no statistical relationships between β-catenin and CD4+ T cells, dendritic cells, macrophages, B cells, or β-catenin protein (All p > 0.05).
FIGURE 5
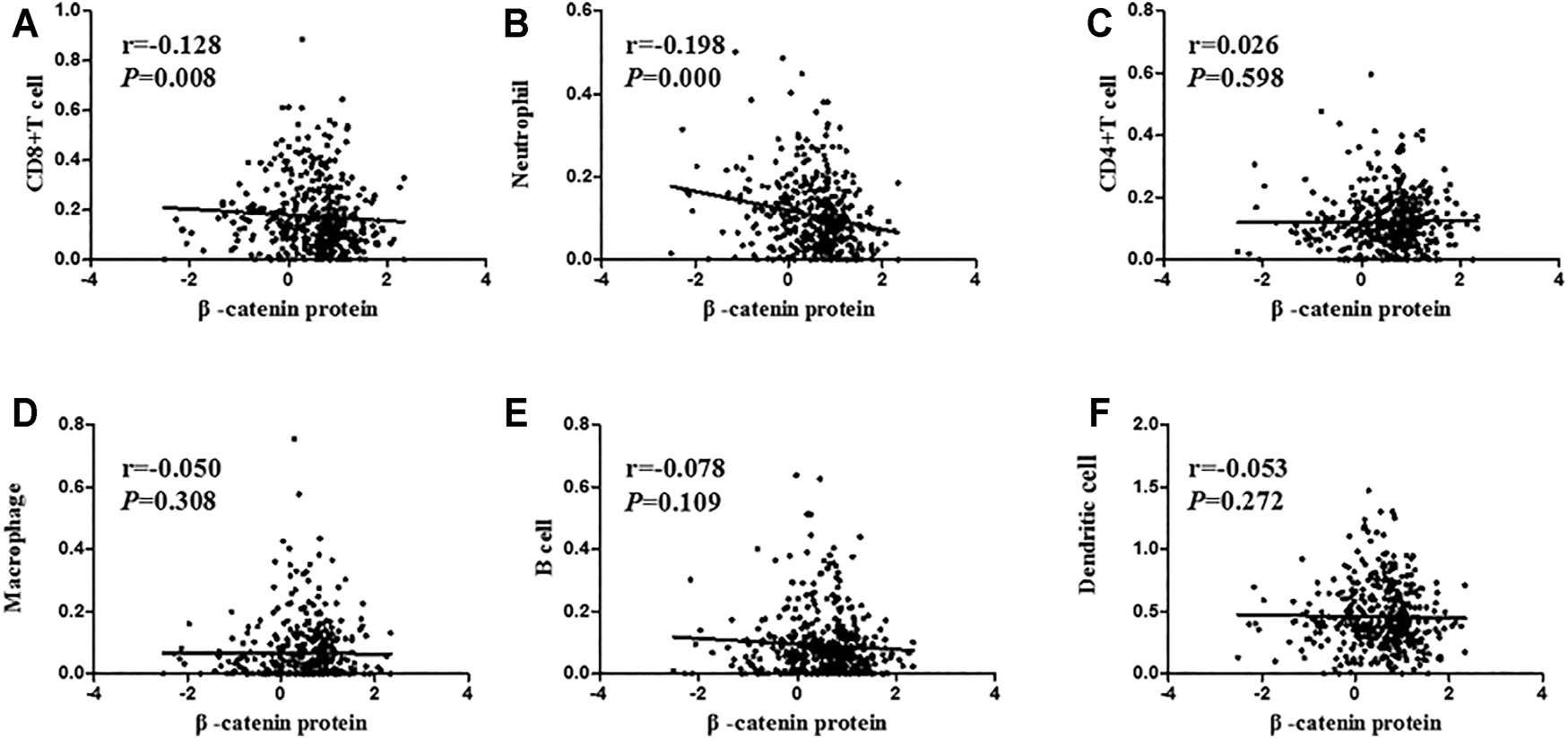
Correlation analysis between β-catenin protein and immune cell infiltration level. β-catenin protein expression had a negative link with CD8+ T cells (A; r = −0.128, p = 0.008) and neutrophils (B; r = −0.198, p < 0.001). β-catenin protein was not related to CD4+ T cells (C; r = 0.026, p = 0.598), macrophages (D; r = −0.050, p = 0.308), B cells (E; r = −0.078, p = 0.109), or dendritic cells (F; r = −0.053, p = 0.272).
More and more evidence has shown the importance of immune and stromal cells in the immune microenvironment [24]. Stromal and immune score was a new method to calculate the infiltrating level of stromal and immune cells. In order to further study the relevance between β-catenin protein level and immune microenvironment, we also assessed the correlation between β-catenin protein and immune scores, including estimate score, immune score, and stromal score. As shown in Figure 6, we found that stromal score (p = 0.045, r = −0.097), immune score (p = 0.024, r = −0.109), and estimate score (p = 0.020, r = −0.113) had a significant negative correlation with β-catenin protein.
FIGURE 6
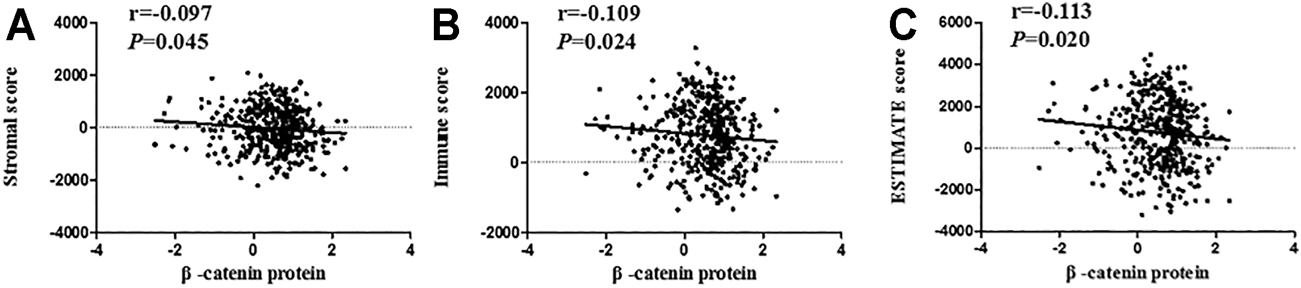
Correlation analysis between β-catenin protein and immune scores. β-catenin protein had a negative relevance with stromal score (A; r = −0.097, p = 0.045), immune score (B; r = −0.109, p = 0.024), and ESTIMATE score (C; r = −0.113, p = 0.020).
Discussion
β-catenin is a multifunctional protein that can interact with cadherin, participate in cell adhesion, and form cytoskeleton tissue [9,10]. Most importantly, it is a critical regulating factor of the classical Wnt pathway. Wnt/β-catenin is abnormally activated in a variety of cancer patients and is closely associated with tumorigenesis, tumor growth, metastasis, and tumor immunity [14–16]. There are some reports on exploring the predictive value of β-catenin in NSCLC [25–27], but its predictive value in a Chinese cohort remains to be further explored. Therefore, this study investigated the connection between β-catenin protein and clinicopathological/prognostic factors, as well as the relevance with infiltrating tumor -immune cells and immune scores. The objective is to discover better predictive targets and to better understand the relation between the Wnt/β-catenin pathway and immune microenvironment.
In previous studies, it has been shown that higher β-catenin protein level was closely related to lymph node metastasis and low survival rate in nasopharyngeal carcinoma patients, which can be used as an indicator of prognosis [28]. Besides, β-catenin expression was reported to be associated with the resistance of NSCLC cells to gefitinib and was important for the distant metastasis of various tumors [29,30]. When the classical Wnt pathway is activated, the cytoplasmic β-catenin is transferred to the nucleus, thereby bringing about the activation of genes related to cancer development [12,13]. Hence, it may be more important to explore the value of abnormal expression of β-catenin protein. In previous literature, reduced membranous β-catenin protein defined as less than 75% of positive cells was related to distant metastasis and worse prognosis in esophageal squamous cell carcinoma patients [31]. Abnormal β-catenin expression defined as more than 10% of cells showing positive in cytoplasm/nuclei or less than 70% of cells showing positive in cytomembrane was correlated with LNM, clinical stages, and survival status of nasopharyngeal carcinoma patients [32]. Also, it has been found that hepatocellular carcinoma patients with β-catenin nuclear accumulation had poor prognosis [33]. Interestingly, β-catenin mRNA expression and its aberrant expression were both increased in LUAD and LUSC. β-catenin mRNA level was also negatively related to overall survival and post-progression survival in LUAD, but not in LUSC. We also discovered aberrant expression of β-catenin protein was associated with gender and histological type, which indicated that β-catenin protein might be used as an index of histological classification. Furthermore, we found that it was identified as a poor prognosis biomarker, which was compatible with previous reports [25–27].
The tumor microenvironment, as the living environment of tumor cells, is composed of various cytokines/chemokines, immune cells, tumor-related fibroblasts, and inflammatory cells [17,18]. ESTIMATE is a new algorithm that estimates the infiltrating grade of immune/stromal cells according to the gene expression profiles of stromal cells and immune cells [24,34]. Therefore, we used TCGA data to search the relevance between β-catenin protein and infiltrating immune cells and thereby to reveal the function of β-catenin protein in tumor immunity and tumor microenvironment. In this research, β-catenin protein was significantly negatively connected with CD8+ T cells and neutrophils. Meanwhile, β-catenin protein was also negatively related to ESTIMATE score, immune score, and stromal score, suggesting that β-catenin protein has a potential function in tumor immunity, which has been found in other studies [35,36]. This is the first study to simultaneously evaluate the relationship between β-catenin protein and multiple immune cells.
In summary, aberrant β-catenin protein expression was dramatically increased in NSCLC and might serve as a poor prognostic biomarker. Most importantly, β-catenin protein might play a key role in tumor immunity and the tumor microenvironment by inhibiting the infiltration of CD8+ T cells and neutrophils.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Review Committee of Central South University, The Second Xiangya Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The National Natural Science Foundation of China (No: 81773218, 81972838, 81703009 and 81472773) and The Natural Science Foundation of Hunan Province (No: 2021JJ30904) supported the work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2021.1609981/full#supplementary-material
Supplementary Figure S1K-M survival analysis of β-catenin protein expression in LUAD and LUSC patients. (A) LUAD patients with aberrant β-catenin protein expression had a shorter OS (p = 0.009). (B) β-catenin protein expression was not related to OS in LUSC ( p =0.349).
References
1.
Siegel RL Miller KD Jemal A . Cancer Statistics, 2019. CA A Cancer J Clin (2019) 69(1):7–34. 10.3322/caac.21551
2.
Zheng H Zhan Y Liu S Lu J Luo J Feng J . The Roles of Tumor-Derived Exosomes in Non-small Cell Lung Cancer and Their Clinical Implications. J Exp Clin Cancer Res (2018) 37(1):226. 10.1186/s13046-018-0901-5
3.
Reck M Heigener DF Mok T Soria J-C Rabe KF . Management of Non-small-cell Lung Cancer: Recent Developments. Lancet (2013) 382(9893):709–19. 10.1016/s0140-6736(13)61502-0
4.
Miller KD Nogueira L Mariotto AB Rowland JH Yabroff KR Alfano CM . Cancer Treatment and Survivorship Statistics, 2019. CA A Cancer J Clin (2019) 69(5):363–85. 10.3322/caac.21565
5.
Zheng H Zhang Y Zhan Y Liu S Lu J Feng J . Prognostic Analysis of Patients with Mutant and Wild-type EGFR Gene Lung Adenocarcinoma. Cancer Manag Res (2019) 11:6139–50. 10.2147/cmar.s200126
6.
Hirsch FR Scagliotti GV Mulshine JL Kwon R Curran WJ Wu Y-L . Lung Cancer: Current Therapies and New Targeted Treatments. Lancet (2017) 389(10066):299–311. 10.1016/s0140-6736(16)30958-8
7.
Nishijima TF Shachar SS Muss HB Tamura K . Patient-Reported Outcomes with PD-1/PD-L1 Inhibitors for Advanced Cancer: A Meta-Analysis. Oncologist (2019) 24(7):e565–e573. 10.1634/theoncologist.2018-0449
8.
Garon EB Hellmann MD Rizvi NA Carcereny E Leighl NB Ahn M-J . Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J Clin Oncol (2019) 37(28):2518–27. 10.1200/jco.19.00934
9.
McCrea P Turck C Gumbiner B . A Homolog of the Armadillo Protein in Drosophila (Plakoglobin) Associated with E-Cadherin. Science (1991) 254(5036):1359–61. 10.1126/science.1962194
10.
Fagotto F . Looking beyond the Wnt Pathway for the Deep Nature of β‐catenin. EMBO Rep (2013) 14(5):422–33. 10.1038/embor.2013.45
11.
Valenta T Hausmann G Basler K . The many Faces and Functions of β-catenin. EMBO J (2012) 31(12):2714–36. 10.1038/emboj.2012.150
12.
Yamaguchi K Nagatoishi S Tsumoto K Furukawa Y . Discovery of Chemical Probes that Suppress Wnt/β‐catenin Signaling through High‐throughput Screening. Cancer Sci (2020) 111(3):783–94. 10.1111/cas.14297
13.
Cui Y Wu X Lin C Zhang X Ye L Ren L . AKIP1 Promotes Early Recurrence of Hepatocellular Carcinoma through Activating the Wnt/β-Catenin/CBP Signaling Pathway. Oncogene (2019) 38(27):5516–29. 10.1038/s41388-019-0807-5
14.
Fodde R Brabletz T . Wnt/β-catenin Signaling in Cancer Stemness and Malignant Behavior. Curr Opin Cel Biol (2007) 19(2):150–8. 10.1016/j.ceb.2007.02.007
15.
Monga SP . β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology (2015) 148(7):1294–310. 10.1053/j.gastro.2015.02.056
16.
Shang S Hua F Hu Z-W . The Regulation of β-catenin Activity and Function in Cancer: Therapeutic Opportunities. Oncotarget (2017) 8(20):33972–89. 10.18632/oncotarget.15687
17.
Albini A Sporn MB . The Tumour Microenvironment as a Target for Chemoprevention. Nat Rev Cancer (2007) 7(2):139–47. 10.1038/nrc2067
18.
Quail DF Joyce JA . Microenvironmental Regulation of Tumor Progression and Metastasis. Nat Med (2013) 19(11):1423–37. 10.1038/nm.3394
19.
Patel S Alam A Pant R Chattopadhyay S . Wnt Signaling and its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front Immunol (2019) 10:2872. 10.3389/fimmu.2019.02872
20.
Gyorffy B Surowiak P Budczies J Lánczky A . Online Survival Analysis Software to Assess the Prognostic Value of Biomarkers Using Transcriptomic Data in Non-small-cell Lung Cancer[J]. PLoS One (2013) 8(12):e82241. 10.1371/journal.pone.0082241
21.
Detterbeck FC Boffa DJ Kim AW Tanoue LT . The Eighth Edition Lung Cancer Stage Classification. Chest (2017) 151(1):193–203. 10.1016/j.chest.2016.10.010
22.
Chen L Xie G Feng J Wen Q Zang H Lu J . Overexpression of FADD and Bcl-XS Proteins as Novel Prognostic Biomarkers for Surgically Resected Non-small Cell Lung Cancer. Cancer Biomark (2021) 30(2):145–54. 10.3233/cbm-190018
23.
Song Y Yang QX Zhang F Meng F Li H Dong Y . Suppression of Nasopharyngeal Carcinoma Cell by Targeting β-catenin Signaling Pathway. Cancer Epidemiol (2012) 36(2):e116–e121. 10.1016/j.canep.2011.11.002
24.
Wang H Wu X Chen Y . Stromal-Immune Score-Based Gene Signature: A Prognosis Stratification Tool in Gastric Cancer. Front Oncol (2019) 9:1212. 10.3389/fonc.2019.01212
25.
Kim H Yoo SB Sun P Jin Y Jheon S Lee CT . Alteration of the E-Cadherin/β-Catenin Complex Is an Independent Poor Prognostic Factor in Lung Adenocarcinoma. Korean J Pathol (2013) 47(1):44–51. 10.4132/koreanjpathol.2013.47.1.44
26.
Muto S Ozaki Y Yamaguchi H Mine H Takagi H Watanabe M . Tumor β-catenin Expression Is Associated with Immune Evasion in Non-small Cell Lung Cancer with High Tumor Mutation burden. Oncol Lett (2021) 21(3):203. 10.3892/ol.2021.12464
27.
Xu X Sun P-L Li J-Z Jheon S Lee C-T Chung J-H . Aberrant Wnt1/β-Catenin Expression Is an Independent Poor Prognostic Marker of Non-Small Cell Lung Cancer After Surgery. J Thorac Oncol (2011) 6(4):716–24. 10.1097/jto.0b013e31820c5189
28.
Sun H Liu M Wu X Yang C Zhang Y Xu Z . Overexpression of N-Cadherin and β-catenin Correlates with Poor Prognosis in Patients with Nasopharyngeal Carcinoma. Oncol Lett (2017) 13(3):1725–30. 10.3892/ol.2017.5645
29.
Fang X Gu P Zhou C Liang A Ren S Liu F . β-Catenin Overexpression Is Associated with Gefitinib Resistance in Non-small Cell Lung Cancer Cells. Pulm Pharmacol Ther (2014) 28(1):41–8. 10.1016/j.pupt.2013.05.005
30.
To SKY Mak ASC Eva Fung YM Che C-M Li S-S Deng W . β-Catenin Downregulates Dicer to Promote Ovarian Cancer Metastasis. Oncogene (2017) 36(43):5927–38. 10.1038/onc.2017.185
31.
Hsu P-K Li AF-Y Wang Y-C Hsieh C-C Huang M-H Hsu W-H . Reduced Membranous β-catenin Protein Expression Is Associated with Metastasis and Poor Prognosis in Squamous Cell Carcinoma of the Esophagus. J Thorac Cardiovasc Surg (2008) 135(5):1029–35. 10.1016/j.jtcvs.2007.11.007
32.
Wang W Wen Q Luo J Chu S Chen L Xu L . Suppression of β-catenin Nuclear Translocation by CGP57380 Decelerates Poor Progression And Potentiates Radiation-Induced Apoptosis in Nasopharyngeal Carcinoma. Theranostics (2017) 7(7):2134–49. 10.7150/thno.17665
33.
Tsai MC Huang CC Wei YC Liu TT Lin MT Yi LN . Combined Chibby and β-Catenin Predicts Clinical Outcomes in Patients with Hepatocellular Carcinoma. Int J Mol Sci (2020) 21(6), 2060. 10.3390/ijms21062060
34.
Yoshihara K Shahmoradgoli M Martínez E Vegesna R Kim H Torres-Garcia W . Inferring Tumour Purity and Stromal and Immune Cell Admixture from Expression Data. Nat Commun (2013) 4:2612. 10.1038/ncomms3612
35.
Pai SG Carneiro BA Mota JM Costa R Leite CA Barroso-Sousa R . Wnt/beta-catenin Pathway: Modulating Anticancer Immune Response. J Hematol Oncol (2017) 10(1):101. 10.1186/s13045-017-0471-6
36.
Li X Xiang Y Li F Yin C Li B Ke X . WNT/β-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front Immunol (2019) 10:2293. 10.3389/fimmu.2019.02293
Summary
Keywords
biomarker, NSCLC, β-catenin, tumor microenvironment, tumor immunity
Citation
Zheng H, Ning Y, Yang Y, Zhan Y, Wang H, Wen Q, Peng J and Fan S (2021) Aberrant Expression of β-Catenin Correlates with Infiltrating Immune Cells and Prognosis in NSCLC. Pathol. Oncol. Res. 27:1609981. doi: 10.3389/pore.2021.1609981
Received
25 July 2021
Accepted
05 October 2021
Published
26 October 2021
Volume
27 - 2021
Edited by
Andrea Ladányi, National Institute of Oncology (NIO), Hungary
Updates
Copyright
© 2021 Zheng, Ning, Yang, Zhan, Wang, Wen, Peng and Fan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songqing Fan, songqingfan@csu.edu.cn; Jinwu Peng, Jinwupeng2005@aliyun.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.