Abstract
The identification of gBRCA1/2 mutations in breast cancer patients is crucial. Successful identification of the mutations has the potential to alter disease treatment and healthcare management of patients whose relatives harbor pathogenic/likely pathogenic (P/LP) variants. In this retrospective analysis, patient- and disease-specific medical data were analyzed in a cohort of breast cancer patients with a known gBRCA1/2 status who were treated between 2019–2021. The prevalence and type of gBRCA1/2 P/LP variants, and their relation to the histopathological data of the cancers, were studied. The presence of one or more clinical criteria leading to germline testing, the outcome of patient management, and family member outcomes were collected. Germline variants were found in 67/259 cases and included 61 P/LP alterations and six “variants of unknown significance” (VUS) of the BRCA1/2 genes. A spectrum of 31 different variants was detected; eight of them occurred in more than one patient, of which three (detected in 26 cases) belonged to the mutations most prevalently detected by the previously used technology in Hungary. The likelihood of revealing a pathogenic gBRCA1/2 mutation increased with the number of risk criteria for germline testing. The presence of three or more risk criteria was predictive for carrying a gBRCA1/2 mutation with an odds ratio (OR) of 10.65 (95% CI 5.20–21.80, p < 0.001). Among the histopathology data, a higher rate of grade 3 or triple negative breast cancer was found among gBRCA1/2 P/LP variant carriers as compared to that in non-carriers. For ultimately revealing a gBRCA1/2 P/LP variant, a positive family history (OR 6.69, 95% CI 1.82–24.64, p = 0.003) and triple negative breast cancer (OR 5.65, 95% CI 2.73–11.71, p < 0.001) were the strongest independent predictive factors. Knowing of gBRCA1/2 alterations meant healthcare management was modified in 86.9% of cases. Germline testing for breast cancer patients, guided by current protocols, is essential for optimizing patient care. Adhering to established clinical criteria facilitates effective patient selection while preventing the unnecessary expansion of testing to average-risk populations. Keywords: BRCA1/2, breast cancer, cancer susceptibility genes, germline testing, medical genetics.
Introduction
Breast cancer is the most common cancer type and a leading cause of death for women worldwide [1]. The majority of breast cancers are sporadic, and the risk of their occurrence is increased by a number of well-known factors such as age, obesity, sedentary lifestyle, smoking, alcohol consumption, hormonal and reproductive history, the diagnosis of certain benign lesions of the breast, or the previous irradiation of the chest [2]. Hereditary breast cancer accounts for only 5%–10% of breast cancers [3] and, in most cases germline mutations of breast cancer, susceptibility genes BRCA1 and BRCA2 are responsible for their development [4]. Other uncommon gene variants with high (TP53, CDH1, PTEN, and PALB2) and low/moderate penetrance (ATM, CHEK2, BRIP1, BARD1, NBN, NF1, RAD51C, RAD51D, and STK11) also increase breast cancer risk; these variants explain 0%–13% of non-BRCA1/2-related hereditary breast cancers, depending on ethnicity [5].
The National Comprehensive Cancer Network (NCCN) Guidelines (v1.2023) recommend testing for high-penetrance breast cancer susceptibility genes in patients diagnosed at age ≤50, those with triple-negative breast cancer (regardless of age), male breast cancer, or Ashkenazi Jewish ancestry. Testing is also indicated for patients with multiple breast cancers (synchronous or metachronous) or a significant family history of associated malignancies. Furthermore, testing guides systemic therapy with PARP inhibitors in both metastatic and adjuvant settings [6]. The detection of cancer susceptibility gene alterations, most importantly BRCA1 and BRCA2 mutations, has become of prime interest in recent years for several reasons. The identification of pathogenic BRCA1 or BRCA2 carriers prompts the implementation of preventive procedures by means of both primary prevention and screening. In addition to the modification of lifestyle factors that add to genetic risk, prophylactic surgical options, such as risk-reducing mastectomy (RRM) and risk-reducing salpingo-oophorectomy (RRSO), are key interventions in both cancer patients and healthy individuals. Chemoprevention with tamoxifen or anastrozole also seems to be effective in gBRCA1/2 mutation carriers. Based on a recently published, international, multicenter study of 5,290 gBRCA1/2 PV-carrier breast cancer patients, after a median follow-up of 8 years, both RRM and RRSO improved overall survival by a HR (Hazard Ratio) = 0.65 (95%CI 0.53–0.78) and HR = 0.58 (95% CI 17.43–18.03), respectively. Although the two interventions were found to be independent, their effects added up [7].
In addition, the oncological treatment of a patient suffering from breast cancer is also directly affected by the detected genetic variation. Genetic testing aids the individualized therapy of breast cancer patients and provides additional treatment options for the affected individuals. In the metastatic setting, both progression-free survival and patient-reported outcome measures were improved in patients treated with the PARP inhibitors olaparib and talazoparib [8, 9]. According to the median 6.1-year follow-up data of the OLYMPIA study, 1 year of adjuvant olaparib therapy significantly improved overall survival, relapse-free survival, and distant disease-free survival in early stage high-risk, HER2-negative breast cancer, irrespective of the hormone receptor status [10]. Furthermore, growing evidence has emerged that incorporation of PARP inhibitor therapy of gBRCA1/2 PV-carrier breast cancer patients in the neoadjuvant setting may increase the rates of pathological complete response (pCR) [11]. The benefit of platinum-based neoadjuvant chemotherapy in the group of gBRCA1/2 PV carriers is still controversial: in a randomized INFORM study, no significant difference was demonstrated between doxorubicin vs. platinum-based chemotherapy combinations [12]. In a meta analysis, however, a trend was shown in favor of platinum-based chemotherapy in this specific patient population [13].
In this study, we collected clinicopathological data from breast cancer patients who underwent germline genetic testing and analyzed the prevalence and distribution of gBRCA1/2 mutations within this Hungarian cohort. The aim was to analyze the clinical criteria for predicting the presence of pathogenic germline alterations in these genes and assess the clinical consequences of a positive test result.
Patients and methods
Study participants and data collection
A retrospective analysis of a prospectively collected medical database of patients who went through genetic counselling and tested positive for hereditary BRCA1/2 mutations apropos of their care for breast cancer at the Department of Oncotherapy, University of Szeged, between January 1, 2019, and 1 January 2022, was performed. This study was approved by the Human Investigation Review Board, University of Szeged, Albert Szent-Györgyi Clinical Centre (#160/2020-SZTE). Patients who met at least one of the following criteria were offered genetic testing: 1. Younger than 50 years of age at the time of the diagnosis of breast cancer; 2. Diagnosed with triple negative breast cancer (estrogen receptor -ER/progesterone receptor -PR/human epidermal growth factor receptor 2 -HER2 negative) at the age of <60 years; 3. ≥1 first- or second-degree relatives with breast cancer at the age of ≤50 years; 4. Bilateral breast cancer at the time of the primary diagnosis or later on; 5. Male breast cancer; or 6. Personal history of ovarian cancer. The germline test result of this patient cohort for the BRCA1/2 genes or a panel of cancer-related genes including BRCA1/2 could have been obtained at any time point of the disease course within the specified time interval of the study.
Patients undergoing germline genetic testing received mandatory genetic counseling prior to sample collection. These consultations were conducted by clinical geneticists at either the Department of Medical Genetics, University of Szeged, or the National Institute of Oncology, Budapest. According to Hungarian legislation, genetic counselling is the exclusive responsibility of clinical geneticists [14]. The standard procedure includes mandatory pre-test genetic counselling and informed consent being given. Likewise, during a post-test consultation, the result of the molecular genetic test has to be explained to the patient by a genetic counsellor. Genetic counselling for cancer patients primarily focuses on therapeutic implications and recurrence risks. Predictive counselling for healthy individuals from hereditary breast and ovarian cancer families usually involves a broader perspective than therapeutic counselling and usually covers individual risks for other malignancies, descendants’ risk to inherit mutations, preventive options, and lifestyle issues. According to the American College of Medical Genetics and Genomics (ACMG) and European Society for Medical Oncology (ESMO) guidelines [15, 16], medical decisions or predictive testing in relatives at risk are not based on the detection of a VUS.
In the database, the following patient- and tumor-related data were recorded: the patients’ age at the time of the first breast cancer diagnosis; their menopausal status; family history of breast or ovarian tumors; histological type; and grade, ER, PR, and HER2 expression of previously diagnosed breast tumors. ER, PR and HER2 expressions were assessed based on immunohistochemistry (IHC). ER and PR were determined as positive if tumor cell nuclei staining was ≥1%. The tumors were considered HER2 negative if the score was 0 or 1+ and positive if the score was 3+. Fluorescence in-situ hybridization (FISH) test was performed and assessed according to the ASCO/CAP HER2 guideline in tumors with IHC 2+ scores. The immunophenotype of the tumor was determined based on ER, PR, and HER2 expression: 1. ER and/or PR positive, HER2 negative; 2. ER and/or PR positive, HER2 positive; 3. ER and PR negative, HER2 positive; and 4. ER, PR, HER2 negative. The stage of the disease at the time of its diagnosis was assessed according to the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system.
gBRCA1/2 gene variant detection and analysis
gDNA was isolated from the patients’ peripheral blood samples with the MagCore Genomic DNA Whole Blood Kit (RBC Bioscience). Next-generation sequencing (NGS) library preparation was carried out using a hybridization-based method, targeting BRCA1/2 genes, including the canonical (+/-30bp) splice donor and acceptor sites and promoter regions (Celemics, Inc.). Sequencing-ready libraries were quality-control checked by Tape Station 4,200 instrument using D5000 ScreenTape (Agilent Technologies USA). NGS was carried out on a NextSeq 550DX sequencing system with NextSeq 500/550 Mid Output Kit v2.5 (300 Cycles) chemistry (Illumina, Inc. United States) or Multiplicom amplicon-based enrichment BRCA MASTR Dx or BRCA MASTR Plus Dx library preparation kit (Agilent Technologies, Santa Clara, CA) and sequenced on the MiSeq Illumina platform (Illumina, San Diego, CA). Bioinformatics analysis was done with the MASTR Reporter software v.1.1 (Agilent Technologies) and by custom bioinformatic analysis pipeline validated to detect single nucleotid variants (SNV) and small insertion/deletion mutations (<40bp)*. Variants are named according to the HGVS guidelines1 using reference sequences NM_007294.4 (BRCA1) and NM_000059.4 (BRCA2). The classification of variants follows the guidelines of ENIGMA (Enigma Consortium | Evidence-based Network for the Interpretation of Germline Mutant Alleles2). Pathogenic variants were confirmed by Sanger sequencing. Benign or likely benign variants were not reported [17, 18].
The result of the genetic test was documented according to the following nomenclature: 1. gBRCA1 mutant with a known BRCA1 pathogenic variant, 2. gBRCA2 mutant with a known BRCA2 pathogenic variant, 3. gBRCA1 or gBRCA2 variant of unknown significance (VUS), or 4. gBRCA1/2 negative if no BRCA1/2 pathogenic variant or VUS was detected. These alterations were analyzed in the context of the previously most prevalently detected gBRCA1/2 mutations in Hungary.
We analyzed the predefined clinicopathological features in the gBRCA1/2 negative and gBRCA1/2 mutation carrier cohorts and compared the clinicopathological characteristics of the gBRCA1 versus gBRCA2 mutation carrier cohorts. The associations between the clinicopathological characteristics of breast cancers and gBRCA1/2 status were investigated. We also recorded the type and number of risk factors indicating genetic counselling based on the above listed criteria and analyzed their role in predicting the gBRCA1/2 mutation carrier status. We evaluated how the result of the genetic test affected the patients' oncological and surgical care and what preventive procedures had been done to reduce cancer risk. In addition, we also recorded the number of cascade BRCA1/2 screenings in families of patients with a germline BRCA1/2 mutation and how many of them had tested positive.
Statistical analyses
Continuous data were expressed as mean ±SD values, if appropriate. Patient- and tumor-related parameters of the gBRCA1/2 mutation carrier and non-carrier groups were compared with independent sample t-test for the continuous and chi-squared test for the categorical variables. Logistic regression models were applied to evaluate the predictive power of various predefined patient-related factors for the presence of gBRCA1/2 mutations. First, binary univariate logistic regression models were used separately, followed by a multivariate logistic regression model to examine the joint effects and interactions. A stepwise procedure was used with a likelihood ratio test. The probability of various cancer events in the presence of pathogenic gBRCA1/2 alterations was examined in a binary univariate logistic regression model. The statistical software IBM SPSS statistics version 29.0 was used for statistical analysis. P-values <0.05 were considered statistically significant.
Results
A total of 259 patients were included in this study, five of whom were males. Twenty-three additional eligible patients during this time period refused to be tested, while four patients who accepted testing were lost to follow-up; hence, these cases were excluded. gBRCA1/2 alterations were found in 67 out of 259 cases and included 61 pathogenic alterations (23.55%) and six VUS (2.31%) of the BRCA1/2 genes; 43/61 (70%) were gBRCA1 while 18/61 (30%) were gBRCA2 mutations. No gBRCA1/2 mutation was found in any of the male patients.
A spectrum of 31 different mutations was detected, 20 in the BRCA1 and 11 in the BRCA2 genes (Table 1). Considering all mutations (n = 31), eight occurred in more than one patient, of which three belonged to the gBRCA alterations previously highly recurrently detected in the Hungarian population. [19]. The group of eight mutations was present in 38/61 (62.3%) of all BRCA1/2 carriers, while founder mutations were detected in 26/61 cases (42.6%).
TABLE 1
| Gene | Nucleotid change | Amino acid change | Mutation type | Exon | Frequency |
|---|---|---|---|---|---|
| BRCA1 | c.181T>G | p.(Cys61Gly) | missense | 5 | 14 |
| BRCA2 | c.9097dupA | p.(Thr3033AsnfsTer11) | insertion/fs | 23 | 5 |
| BRCA1 | c.5266dupC | p.(Gln1756Pro fsTer74) | insertion/fs | 20 | 6 |
| BRCA1 | del(ex21-22) | - | deletion | 21-22 | 5 |
| BRCA2 | c.9117G>A | p.(Pro3039=) | silent | 24 | 2 |
| BRCA2 | c.9371A>T | p.(Asn3124Ile) | missense | 25 | 2 |
| BRCA1 | del(ex24) | - | deletion | 24 | 2 |
| BRCA2 | c.7913_7917delTTCCT | p.(Phe2638Ter) | nonsense | 17 | 2 |
| BRCA1 | c.66_67delAG | p.(Glu23ValfsTer17) | deletion/fs | 2 | 1 |
| BRCA1 | c.3700_3704delGTAAA | p.(Val1234GlnfsTer8) | deletion/fs | 11 | 1 |
| BRCA1 | c.5278-492_5407-128delins236, del(ex21-22) | p.(Ile1760_Thr1802) | exon deletion | 21-22 | 1 |
| BRCA1 | c.5545G>T | p.(Glu1849Ter) | nonsense | 24 | 1 |
| BRCA1 | c.5030_5033del CTAA | p.(Thr1677IlefsTer2) | deletion/fs | 17 | 1 |
| BRCA1 | c.115T>C | p.(Cys39Arg) | missense | 3 | 1 |
| BRCA1 | c.2296_2297delAG | p.(Ser766fsTer) | nonsense | 11 | 1 |
| BRCA1 | c.227_228delGT | p.(Ser76AsnfsTer4) | deletion/fs | 6 | 1 |
| BRCA1 | c.3756_3759delGTCT | p.(Ser1253ArgfsTer10) | deletion/fs | 11 | 1 |
| BRCA1 | c.2329delT | p.(Tyr777MetfsTer15) | deletion/fs | 11 | 1 |
| BRCA1 | c.3018_3021delTTCA | p.(His1006GlnfsTer17) | deletion/fs | 11 | 1 |
| BRCA1 | dup(ex13) | duplication | 13 | 1 | |
| BRCA1 | c.1016dupA | p.(Val340GlyfsTer6) | insertion/fs | 11 | 1 |
| BRCA1 | c.5251C>T | p.(Arg1751Ter) | nonsense | 20 | 1 |
| BRCA1 | c.843_846delCTCA | p.(Ser282TyrfsTer15) | deletion/fs | 11 | 1 |
| BRCA2 | c.6656C>G | p.(Ser2219Ter) | nonsense | 11 | 1 |
| BRCA2 | c.8755-1G>A | splice acceptor variant | IVS21 | 1 | |
| BRCA2 | c.7069_7070delCT | p.(Leu2357ValfsTer2) | deletion/fs | 14 | 1 |
| BRCA2 | c.7595_7596insTT | p.(Ala2534LeufsTer18) | insertion/fs | 15 | 1 |
| BRCA2 | c.1296_1297delGA | p.(Asn433GlnfsTer18) | deletion/fs | 10 | 1 |
| BRCA2 | c.6644_6647delACTC | p.(Tyr2215SerfsTer13) | deletion/fs | 11 | 1 |
| BRCA2 | c.9976A>T | p.(Lys3326Ter) | nonsense | 27 | 1 |
| BRCA1 | c.5161C>T | p.Gln1721Ter | nonsence | 19 | 1 |
Types and distribution of pathological gBRCA1/2 variants identified in 61 patients
Due to limited access to genetic testing at our university, these tests had to be performed in two different institutes: the Department of Medical Genetics, University of Szeged, and the Department of Molecular Genetics, National Institute of Oncology, Budapest. In Szeged, testing costs were initially covered by patients out-of-pocket, followed by a private foundation, and subsequently by the National Health Insurance Fund of Hungary. In contrast, all testing in Budapest was funded by the National Health Insurance Fund from the beginning. In 250 cases, genetic tests were restricted to the BRCA1/2 genes, while in nine cases a comprehensive panel of 113 genes related to hereditary cancers was tested. No difference was found between the number or distribution of pathogenic BRCA variants according to the laboratory where the determination was carried out.
The six cases with BRCA1/2 VUS were excluded from further analyses.
The mean age at the diagnosis of breast cancer of the cohort of 253 patients was 44.6 ± 10.03 (range, 25–78) years.
The distribution of cases according to the reason for genetic counselling is represented in Figure 1. While in the gBRCA1/2 non-carrier group most patients reported one or two clinical criteria only (n = 176; 91.7%), in the gBRCA1/2 carrier group half of the patients had three or more of them (n = 30; 49.2%) present. The number of risk factors was significantly associated with the finding of gBRCA1/2 mutation. The presence of three or more risk criteria was predictive for carrying a gBRCA1/2 mutation with an odds ratio (OR) of 10.65 [95% confidence interval (CI), 5.20–21.80; p < 0.001] (Table 2).
FIGURE 1
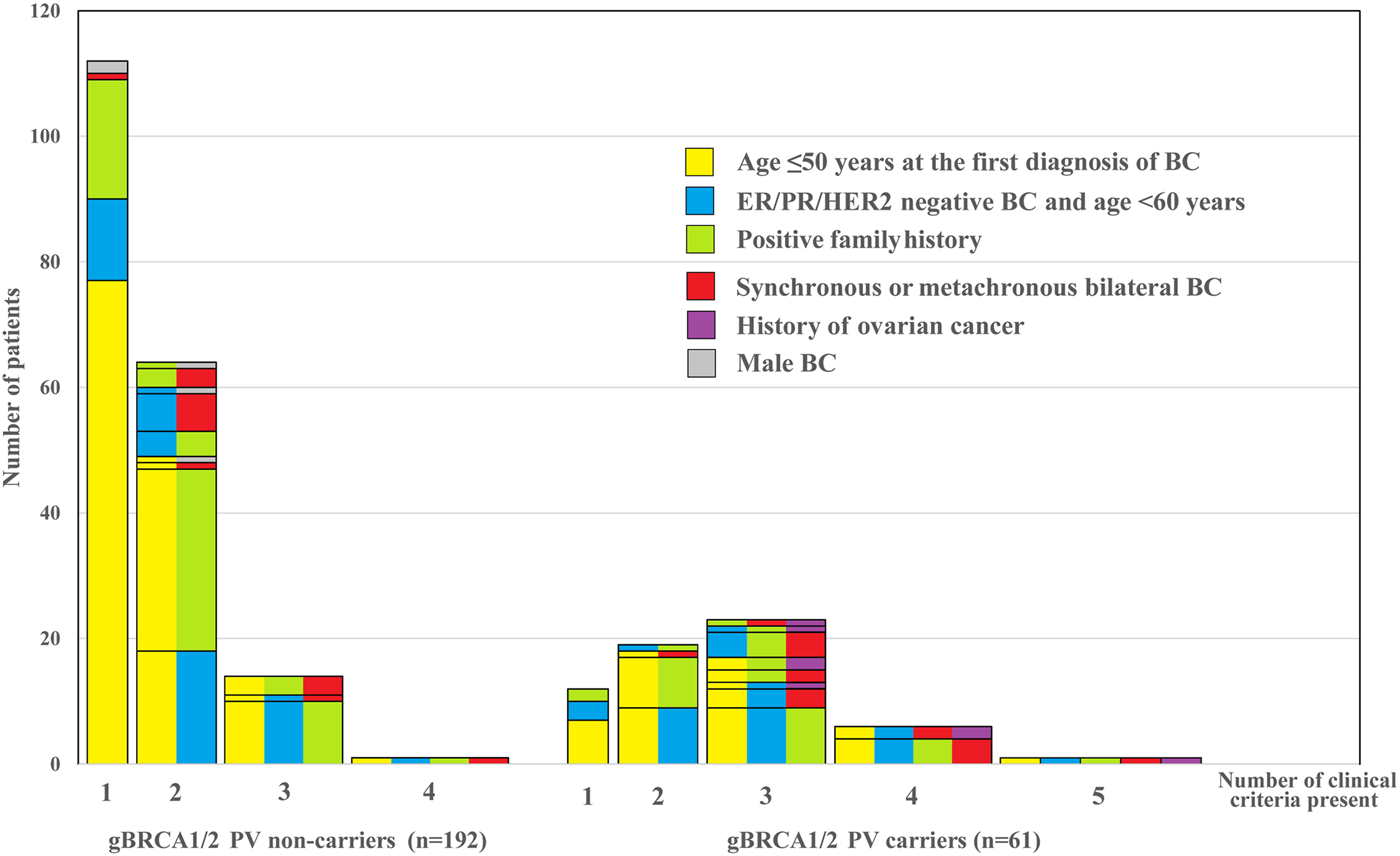
Clinical criteria and their combination suggesting the need of germline testing in a cohort of 253 cases according to the outcome of testing. Bars represent the number of patients with the presence of one or more (1–5) such factors in various combinations according to the test result (pathogenic gBRCA1/2 mutation non-carriers vs. carriers). The color combinations indicate the specific variety of clinical criteria and, cases with identical combinations add up in one rectangle. Note that the bars from left to right in the two groups, respectively, indicate an increasing number of risk criteria that one by one may justify germline genetic testing.
TABLE 2
| Parameter | BRCA1/2 P/LP non-carriers n = 192 (%) | BRCA1/2 P/LP carriers n = 61 (%) | OR univariate (95% CI) | OR multivariate (95% CI) |
|---|---|---|---|---|
| age ≤50 years | 141 (73.4) | 49 (80.3) | 1.48 (0.73-3.01) p=0.280 | Excluded |
| ER/PR/HER2 negative | 55 (28.6) | 40 (65.6) | 4.75 (2.57-8.77) p < 0.001 | 5.65 (2.73-11.71) p < 0.001 |
| Positive family history –second degree relatives only | 32 (16.7) | 10 (16.4%) | 1.76 (0.75-4.10) p=0.193 | 1.47 (0.58-3.72) p=0.422 |
| Positive family history – first degree relatives only | 30 (15.6) | 21 (34.4) | 3.93 (1.90-8.13) p < 0.001 | 5.86 (2.53-13.56) p < 0.001 |
| Positive family history – first and second degree relatives | 8 (4.2) | 6 (9.8) | 4.21 (1.33-13.39) p = 0.015 | 6.69 (1.82-24.61) p = 0.003 |
| Syncronous or metachronous bilateral breast cancer | 16 (8.3) | 17 (27.9) | 4.25 (1.99-9.07) p < 0.001 | 2.76 (1.17-6.55) p = 0.021 |
| Ovarian cancer in personal history | 0 (0.0) | 7 (11.5) | Excluded | Excluded |
| Number of risk factors ≥3 | 16 (8.3) | 30 (49.2) | 10.65 (5.20-21.80) p < 0.001 | Excluded |
Univariate and multivariate logistic regression analysis of the gBRCA1/2 mutation carrier status and various patient- and tumor-related parameters
Abbreviations: ER, estrogen receptor; PR, progesteron receptor; HER2, Human Epidermal Growth Factor Receptor 2.
The associations between the clinicopathological variables and the gBRCA1/2 mutational status of patients are summarized in Table 3. There was no significant difference among the gBRCA1/2 mutation non-carriers and gBRCA1/2 mutation carriers regarding the age, proportion of patients under 50 years of age, or menostatus. The histological tumor type did not differ significantly; most of the tumors in both groups were invasive adenocarcinomas of no specific type. The tumor size, nodal status, and tumor stage showed no differences. However, there was a significant difference in the distribution of histopathological grades among the two groups, with a higher rate of grade 3 cancers among the gBRCA1/2 mutant cases. Likewise, there was a significantly higher rate of triple-negative phenotype in the gBRCA1/2 mutant cohort. These specific patient- and disease-related characteristics were also investigated according to the type of BRCA1/2 mutation. There was no difference regarding the age between the gBRCA1 versus gBRCA2 carrier patients, with the mean age at the time of diagnosis being 43.26 ± 8.92 (range, 29–60) versus 41.05 ± 6.70 (range, 28–55) years, respectively. Histopathological grade was higher in cancers of BRCA1 mutation carriers than that of BRCA2 mutation carriers. Furthermore, 82.8% of gBRCA1 mutation carriers had triple-negative breast cancer while that subtype occurred in 5.9% of the gBRCA2 mutation carriers only. More than two-thirds of the patients carrying a BRCA2 mutation had hormone receptor-positive and HER2-negative tumors (Table 4).
TABLE 3
| Characteristics | gBRCA1/2 P/LP non-carriers | gBRCA1/2 P/LP carriers | p | |
|---|---|---|---|---|
| Patients | n = 192 (%) | n = 61 (%) | ||
| Mean age (range), years | 45.3 (25-78) | 42.6 (28-63) | p = 0.068 | |
| Age group (%) | <40 years | 63 (32.8) | 29 (47.5) | p = 0.101 |
| 40–50 years | 78 (40.6) | 21 (34.4) | ||
| >50 years | 51 (26.6) | 11 (18.1) | ||
| Menopausal status/gender (%) | premenopausal | 134 (69.8) | 49 (80.3) | p = 0.181 |
| postmenopausal | 53 (27.6) | 12 (19.7) | ||
| male | 5 (2.6) | 0 | ||
| All cancers | n = 208 (%) | n = 78 (%) | ||
| Breast cancer histologic type | DCIS | 12 (5.8) | 1 (1.3) | p = 0.141 |
| NST | 163 (78.4) | 67 (87.0) | ||
| ILC | 20 (9.6) | 3 (3.9) | ||
| other | 13 (6.3) | 6 (7.8) | ||
| not available | 1 | |||
| TNM tumor size (%) | Tis | 12 (5.8) | 1 (1.3) | p = 0.365 |
| T1 | 73 (35.2) | 25 (33.3) | ||
| T2 | 91 (44.0) | 38 (50.8) | ||
| T3 | 23 (11.1) | 10 (13.3) | ||
| T4 | 8 (3.9) | 1 (1.3) | ||
| not available | 1 | 3 | ||
| TNM nodal status (%) | N0 | 124 (60.2) | 46 (61.4) | p = 0.255 |
| N1 | 68 (33.0) | 21 (28.0) | ||
| N2 | 8 (3.9) | 7 (9.3) | ||
| N3 | 6 (2.9) | 1 (1.3) | ||
| not available | 2 | 3 | ||
| TNM stage (%) | Stage 0 | 12 (5.8) | 1 (1.3) | p = 0.059 |
| Stage 1 | 57 (27.7) | 19 (24.4) | ||
| Stage 2 | 103 (50.0) | 34 (43.6) | ||
| Stage 3 | 20 (9.7) | 13 (16.7) | ||
| Stage 4 | 14 (6.8) | 7 (9.0) | ||
| not known | 2 | 4 | ||
| Invasive cancers | n = 196 (%) | n = 77 (%) | ||
| Histological grade | 1 | 18 (9.4) | 1 (1.4) | p = 0.006 |
| 2 | 72 (37.7) | 18 (24.6) | ||
| 3 | 101 (52.9) | 54 (74.0) | ||
| not available | 5 | 4 | ||
| IHC subtype groups (%) | ER and/or PR+, HER2- | 115 (58.7) | 23 (30.7) | <0.001 |
| ER and/or PR+, HER2+ | 16 (8.2) | 1 (1.3) | ||
| ER and PR-, HER2+ | 7 (3.6) | 2 (2.7) | ||
| ER and PR-, HER2- | 58 (29.6) | 49 (65.3) | ||
| not available | 2 | |||
Patient- and tumor-related characteristics in the entire study population (note that all cancers are included in bilateral breast cancer cases)
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, Human Epidermal Growth Factor Receptor 2; IHC, immunohistochemistry; ILC, invasive lobular cancer; NST, adenocarcinoma of the breast of no special type; PR, progesteron receptor; TNM, tumor-node-metastasis.
TABLE 4
| Characteristics | gBRCA1 P/LP carriers n=43 (%) | gBRCA2 P/LP carriers n=18 (%) | ||
|---|---|---|---|---|
| Mean age ± SD, years | 43.26 ± 8.92 | 41.05 ± 6.70 | p = 0.352 | |
| Invasive cancers | n = 59 (%) | n = 18 (%) | ||
| Histological grade (%) | 1 | 0 (0) | 1 (6.3) | p = 0.065 |
| 2 | 12 (21.1) | 6 (37.5) | ||
| 3 | 45 (78.9) | 9 (56.2) | ||
| not available | 2 | 2 | ||
| IHC subtype, (invasive cancers,%) | ER and/or PR+, HER2- | 8 (13.8) | 15 (88.2) | p < 0.001 |
| ER and PR-, HER2+ | 1 (1.7) | 1 (5.9) | ||
| ER and/or PR+, HER2+ | 1 (1.7) | 0 (0.0) | ||
| ER and PR-, HER2- | 48 (82.8) | 1 (5.9) | ||
| not available | 1 | 1 | ||
Selected patient- and tumor-related characteristics according to the gBRCA1 versus gBRCA2 P/LP carrier status (note that in bilateral cases all cancers are included independently of the time of diagnosis during the course of the disease).
Abbreviations: ER, estrogen receptor; HER2, Human Epidermal Growth Factor Receptor 2; IHC, immunohistochemistry; PR, progesteron receptor.
Next, we evaluated the association between the indications for genetic testing and the resulting genetic findings. A history of breast cancer in first-degree relatives exclusively was elicited from 51 (20.2%) patients. Another 42 patients (16.6%) reported a second-degree relative without affected first-degree relatives. In addition, 14 patients had breast cancer in both first-degree and second-degree relatives. The incidence of gBRCA1/2 mutation was the highest among the patients with a personal history of ovarian cancer (7/7, 100%), followed by the occurrence of a synchronous bilateral breast cancer (SBBC) or metachronous bilateral breast cancer (MBBC) (17/33, 51.5%). A family history of breast cancer in first- and second-degree relatives (6/14, 42.9%) or in first-degree relatives exclusively (21/51, 41.2%), as well as the triple-negative phenotype of breast cancer (40/95, 42.1%), also indicated a high prevalence of gBRCA1/2 mutation. Further analyses were performed in logistic regression models. In univariate analysis, the strongest predictive effect on the detection of a pathogenic gBRCA1/2 mutation was shown for a triple-negative phenotype with an OR of 4.75 (95% CI 2.57–8.77, p < 0.001) and the presence of ≥3 predefined factors with an OR of 10.65 (95% CI 5.20–21.80, p < 0.001) (Table 2). In multivariate analysis, a positive family history (OR 6.69, 95% CI 1.82–24.61, p = 0.003), triple-negative breast cancer (OR 5.65, 95% CI 2.73–11.71, p < 0.001), and bilateral breast cancer (OR 2.76, 95% 1.17–6.55, p = 0.021) remained independent predictive factors (Table 2).
Knowing of gBRCA1/2 alterations meant healthcare management was modified in 43/61 (70.5%) of cases for one or more of the following aspects: a platinum agent was integrated into the neoadjuvant chemotherapy regimen in 12 cases, the adjuvant PARP inhibitor olaparib therapy was recommended for two patients, while 13 patients subsequently received palliative PARP inhibitor therapy for metastatic disease. The original surgical plan was revised by the result of the genetic test for 13 patients (mastectomy was performed instead of excision). Altogether 13 patients underwent risk-reducing contralateral mastectomy, and 16 patients made a decision on prophylactic salpingo-oophorectomy. With regard to the relatives, cascade BRCA1/2 screening was performed in 25 families; in 19 families, 25 germline pathogenic variants were identified. There were only 10 cases (16.4%) in which the knowledge of the gBRCA1/2 PV carrier status neither influenced the systemic therapy nor led to prophylactic surgery or prompted a cascade screening of the family.
Discussion
In this retrospective study, we analyzed the initial implementation of genetic testing in routine clinical practice. Among 259 cases preselected based on high-risk indicators, we identified a 23.5% rate of pathogenic mutations. We evaluated the associations between the clinicopathological features and the ultimate finding of gBRCA1 or gBRCA2 mutations: a second ovarian cancer, the diagnosis of SBBC or MBBC, or the simultaneous presence of multiple clinical criteria besides having a triple negative breast cancer phenotype at the age of <60 years or a positive family history were identified as the strongest predictive factors.
The identification of gBRCA1/2 mutations in breast cancer patients is fundamental. Successful identification of the mutations has the potential to alter disease treatment and the future healthcare management of patients whose relatives harbor pathogenic/likely pathogenic (P/LP) variants. A cascade screening extended to family members may play a significant role in cancer prevention [20]. The reception of germline genetic testing has immensely changed in the past few years. Both the evolution of professional guidelines and increased openness from the side of patients and society have occurred. True accelerator factors for these developments have been the registration of the targeted agent PARP inhibitors 8 and 3 years ago for metastatic and early breast cancer, respectively, together with the widening access to genomic testing and the “Angelina Jolie effect” [21].
Current NCCN guidelines recommend testing for high-penetrance breast cancer susceptibility genes in all patients diagnosed at age ≤50. Testing is also indicated to guide systemic therapy with PARP inhibitors or regardless of age in cases of triple-negative breast cancer, multiple primary breast cancers, or a positive family history [20]. The ASCO guideline suggests an age limit of ≤65 years for universal testing of all breast cancer patients with stage 1–4 disease and the examination of older patients if any positive impact could be expected on their systemic therapy or if they had triple negative cancer, a positive family history, an Ashkenazi Jewish ancestry, or if they were males [22]. Finally, the American Society of Breast Surgeons recommends testing of at least gBRCA1/2 and gPALB2 (but a larger panel of genes if the alteration of other genes is suspected) in all breast cancer cases [23]. Notably, a significant prevalence of germline alterations was detected among nearly 1,000 breast cancer patients using a wide panel who did not meet screening criteria [24]. In fact, the global limitations in clinical geneticist and laboratory capacities at present restrict the extension of service to all breast cancer patients. The use of comprehensive gene panels would render even more burden on that insufficient network by revealing at least double the pathogenic alterations, although with lower penetrance than that of the gBRCA1/2 gene mutations [25, 26]. Another consequence that would encumber both the individual and the healthcare system is the identification of VUS that must be followed for its correct evaluation in view of newly evolving data and experiences. Nevertheless, efforts made in this field will result in better survival of mutation carriers: targeted breast magnetic resonance imaging (MRI) screening results in improved survival among healthy individuals carrying gBRCA1/2 P/LP variants [27] and breast cancer-specific mortality is significantly improved if genetic testing is performed before the occurrence of breast cancer [28]. Moreover, gBRCA1/2 mutant breast cancer patients show improved survival if they undergo a risk-reducing mastectomy and/or bilateral salpingo-oophorectomy [7]. The use of PARP inhibitors improves survival as well [8, 29, 30]. It is predicted that these various interventions based on the knowledge of high-penetrance breast cancer susceptibility gene mutation carrier status will be increasingly utilized in both breast cancer and high-risk healthy individuals. It has been demonstrated that both the use of comprehensive cancer gene panels (MGP) and extending testing to a wider population renders this service more cost-effective [31], even in Hungary, where the largest genomic center showed that using a multigene panel testing decreased both the cost and turnaround time significantly [32].
In 2018, we introduced systematic genetic testing for at-risk individuals, implementing the NCCN guidelines for patient selection that were in effect at that time. Although initially we were faced with many difficulties, now all patients or healthy individuals in need in Hungary have potential access to genetic testing with varying turnaround times. Patients have recently become more knowledgeable, probably due to media and informal communication: while initially some patients refused examination, nowadays most patients actively seek genetic testing. The correct information and interpretation of findings by the multidisciplinary team and sufficient time for decision making are key elements for correct patient management. The findings from the Surveillance, Epidemiology, and End Results (SEER) database analysis by Kurian et al. increased attention to a trend of care less aligned with guidelines (i.e., less radiotherapy and more chemotherapies were delivered) of breast cancer patients who carried a pathologic germline mutation as opposed to non-carriers, even if a less penetrant alteration was present [33]. We believe that the practice of germline breast cancer susceptibility gene testing is rapidly evolving and both providers and consumers will make an impact on it. At present, much could be gained through the provision of systematic screening of high-risk breast cancer individuals with no gaps in access.
Universal testing for all breast cancer patients, regardless of clinical data or family history, is an emerging approach that increases both testing access and the detection rate of PVs. Two robust microsimulation studies, one using UK and US databases [34] and the other just US databases [35], have demonstrated that the universal testing of BRCA1/2 and PALB2 in breast cancer patients have major effects on cancer prevention and treatment and is extremely cost-effective. A rapid transformation of routine practice, including the widening of the patient population tested and genes examined, is foreseen in the future.
Among our high-risk patients, the prevalence of gBRCA1 and gBRCA2 mutations was 16.6% and 6.9%, respectively. In a similar study conducted in Romania in a cohort satisfying the international criteria for testing, similar rates of 12% and 6.8% of gBRCA1 and gBRCA2 mutations were found [36]. Likewise, in a recently published Turkish paper, 13.6% gBRCA1 and 12.3% gBRCA2 mutation rates were detected [37]. In a recent Hungarian prospective study analyzing 463 patients, Nagy et al. showed that 96 patients (20.7%) harbored P/LP variants, 67 in high-penetrance genes (56 in BRCA1/2) and 29 in moderate-penetrance genes. P/LP variants in this cohort belonged to the BRCA1 and BRCA2 genes in 6.9% and 5.2%, respectively. The use of an extended breast cancer panel doubled the detection rate from 12.1% (56/463) to 20.7% (96/463) as compared to BRCA1/2 testing only [38].
According to literature data, 4%–40% of men diagnosed with breast cancer harbor a gBRCA2 mutation, while the detection of a gBRCA1 PV is much rarer; the presence of gBRCA2 PV indicates a lifetime risk for breast cancer of 8.9% [39, 40]. Notably, due to the lack of large studies, no exact prevalence data are known. In this specific group, the median age at diagnosis is 62 years, most of the cancers are of high grade, ER, PR positive, and HER2 negative, and the disease is of poor outcome; in contrast with their female counterparts, the lobular histology is unusual [40]. Although the described age and histology features were typical among our male breast cancer patients, no gBRCA1/2 mutation was detected among them [39]. We think that the insufficient case number led to the absence of gBRCA1/2 PV among our male breast cancer patients. In fact, we had 11 male breast cancer patients whom we offered germline genetic testing to during the time period specified, however, only five patients participated. Our findings are consistent with those of Cheng et al., who recently reported lower rates of genetic testing compliance compared to female patients.
In this research, only 26/61 (42.6%) of the gBRCA1/2 variants belonged to the well-known, recurrent pathogenic alterations for Hungary, demonstrating the clinical relevance of comprehensive full-length testing of the BRCA1 and BRCA2 genes over the testing of hotspot regions. In our study, although the proportion of patients <40 years of age was higher in gBRCA1/2 mutation carriers versus non-carriers (47.5% versus 32.8%), there was no difference between the mean age of the two groups. One explanation for this finding could be that a young age at diagnosis itself was an inclusion criterion. Notably, by performing systematic germline breast cancer gene alteration screening, various pathological variants including that of the BRCA1/2 genes were revealed in older patients too [25]. Penetrance of the disease, and differences among ethnicities and races, may also have an impact [41–43].
There were no significant differences between the gBRCA1/2 carriers and non-carriers regarding the cancer-related data. Most studies have reported that the gBRCA mutation status has no influence on tumor size or lymph node status [37, 44–48]. Nonetheless, some studies reported larger tumor sizes and a higher percentage of lymph node metastases in gBRCA mutation carriers [49–51]. Regarding the histological type, several studies have confirmed that the majority of invasive breast cancers are breast adenocarcinoma of no special type; a higher frequency of lobular and tubular cancers were reported in gBRCA2-associated tumors [52, 53]. Yadav et al, specifically focusing on ILC, found that pathological variants in the ATM, BRCA2, CDH1, CHEK2, and PALB2 genes increased the risk of that histological type while those of the gBRCA1 gene did not [54]. In line with this finding, our three ILC patients in the gBRCA1/2 group carried the alteration of the BRCA2 gene.
Tumors arising in gBRCA1 mutation carriers are more often poorly differentiated and have a higher histopathological grade than those with a gBRCA2 mutation or without gBRCA1/2 mutations [37, 44, 55, 56]. We also found that the grade was higher in gBRCA1-related breast cancers than in gBRCA2-related breast cancers and gBRCA1/2 mutation non-carriers.
Triple-negative breast cancer represents 10%–15% of sporadic invasive breast carcinomas. Nevertheless, among gBRCA1 and gBRCA2 mutation carriers, 50%–88% and 14.6%–34% of the breast tumors were triple negative, respectively [37, 55, 57]. Our results are in accordance with these findings. Although genetic testing was recommended for triple-negative breast cancer patients with age restriction during the time period we studied, updated guidelines suggest the indication criteria for genetic counselling should be expanded to include all triple-negative breast cancer patients, regardless of age. Although no ER-low positive breast cancers were identified in our study, we consider it important to emphasize that these tumors are more similar to triple-negative breast cancers in terms of both their clinical behavior and genetic characteristics. According to data from the literature, the proportion of gBRCA1/2 PV carriers among patients with ER-low positive tumors is comparable to that observed in the triple-negative subgroup; hence, genetic testing is considered justified in ER-low cases [58].
Our findings support that, beyond positive family history, bilateral breast cancer, and diagnosis of a second ovarian tumor, specific pathological features of the breast tumor may serve as valuable indicators in identifying patients at elevated risk for hereditary breast cancer. Our results confirm that the aspects indicating genetic counseling, as defined by international guidelines, are associated with a higher likelihood of identifying hereditary genetic alterations as a risk factor for breast cancer compared to an unselected breast cancer population, thereby supporting the cost-effective implementation of genetic testing in routine clinical practice.
Conclusion
The germline testing of breast cancer patients based on actual guidelines is necessary for optimizing the care of related individuals; considering well-established clinical criteria both promotes patient selection for genetic testing and prevents the unnecessary testing of broader populations of average risk.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Human Investigation Review Board, University of Szeged, Albert Szent- Györgyi Clinical Centre (#160/2020-SZTE). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in the germline genetic testing.
Author contributions
Conceptualization, AN and ZK; methodology, AN, ÁD, DS, RK, ZK, AP, HB, JP, EH, KP, ME, and BC software, ZV; validation, AN and ZV; formal analysis, ZV; investigation, AN, RK, ÁD, DS, and MI resources, AP, HB, JP, EH, KP, ME, BC, and RT data curation, AN, RK, ÁD, DS, MI, ZV, and RT writing – original draft preparation, AN; writing – review and editing, AP, JO, and ZK; visualization, ZV; supervision, JO and ZK. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declared that financial support was received for this work and/or its publication. The authors declare that this study received funding from Kelemen János Alapítvány a Szegedi Daganatgyógyításért foundation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
Authors ME, KP, and BC were employed by Delta Bio 2000 Ltd.
The remaining author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
1.
SungHFerlayJSiegelRLLaversanneMSoerjomataramIJemalAet alGlobal cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. 10.3322/caac.21660
2.
FakhriNChadMALahkimMHouariADehbiHBelmoudenAet alRisk factors for breast cancer in women: an update review. Med Oncol (2022) 39(12):197. 10.1007/s12032-022-01804-x
3.
RippergerTGadzickiDMeindlASchlegelbergerB. Breast cancer susceptibility: current knowledge and implications for genetic counselling. Eur J Hum Genet (2009) 17(6):722–31. 10.1038/ejhg.2008.212
4.
FackenthalJDOlopadeOI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer (2007) 7(12):937–48. 10.1038/nrc2054
5.
MahdaviMNassiriMKooshyarMMVakili-AzghandiMAvanASandryRet alHereditary breast cancer; Genetic penetrance and current status with BRCA. J Cell Physiol (2019) 234(5):5741–50. 10.1002/jcp.27464
6.
BeversTBNiellBLBakerJLBennettDLBonaccioECampMSet alNCCN guidelines® insights: breast cancer screening and diagnosis, version 1.2023. J Natl Compr Canc Netw (2023) 21(9):900–9. 10.6004/jnccn.2023.0046
7.
BlondeauxESonnenblickAAgostinettoEBasRKimHJFranzoiMAet alAssociation between risk-reducing surgeries and survival in young BRCA carriers with breast cancer: an international cohort study. Lancet Oncol (2025) 26(6):759–70. 10.1016/S1470-2045(25)00152-4
8.
RobsonMEImSASenkusEXuBDomchekSMMasudaNet alOlympiAD extended follow-up for overall survival and safety: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Eur J Cancer (2023) 184:39–47. 10.1016/j.ejca.2023.01.031
9.
PötterRTanderupKKirisitsCde LeeuwAKirchheinerKNoutRet alThe EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol (2018) 9:48–60. 10.1016/j.ctro.2018.01.001
10.
GarberJEet alPre-specified analyses of IDFS, DDFS and OS 10 years from first patient. In: (FPI) in the OlympiA trial of adjuvant olaparib in germline BRCA1/2 mutation-associated breast cancer, SABCS2024 presentation (2024). Available online at: https://medcomms.project.mimsit.net/wp-content/uploads/sites/6/2024/12/OlympiA-presentation-at-SABCS2024.pdf.
11.
JiaXWangKZhuoQZhaoZLiM. PARP inhibitor for neoadjuvant therapy in HER2-Negative breast cancer: a systematic review and meta-analysis of efficacy and safety. Clin Breast Cancer (2024) 24(5):392–8.e3. 10.1016/j.clbc.2024.02.020
12.
TungNArunBHackerMRHofstatterEToppmeyerDLIsakoffSJet alTBCRC 031: randomized phase II study of neoadjuvant cisplatin Versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-Negative breast cancer (the INFORM trial). J Clin Oncol (2020) 38(14):1539–48. 10.1200/JCO.19.03292
13.
CarameloOSilvaCCarameloFFrutuosoCAlmeida-SantosT. The effect of neoadjuvant platinum-based chemotherapy in BRCA mutated triple negative breast cancers -systematic review and meta-analysis. Hered Cancer Clin Pract (2019) 17:11. 10.1186/s13053-019-0111-y
14.
Klinikai genetika tagozat Az emberi erőforrások minisztériuma egészségügyi szakmai irányelve A genetikai tanácsadásról (2020). Available online at: http://www.hbcs.hu/uploads/jogszabaly/3278/fajlok/2020_EuK_20_szam_EMMI_szakmai_iranyelv_2.pdf.
15.
MillerDTLeeKAbul-HusnNSAmendolaLMBrothersKChungWKet alACMG SF v3.1 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American college of medical genetics and genomics (ACMG). Genet Med (2022) 24(7):1407–14. 10.1016/j.gim.2022.04.006
16.
van de HaarJRoepmanPAndreFBalmañaJCastroEChakravartyDet alESMO recommendations on clinical reporting of genomic test results for solid cancers. Ann Oncol (2024) 35(11):954–67. 10.1016/j.annonc.2024.06.018
17.
EnyediMZJaksaGPintérLSükösdFGyurisZHajduAet alSimultaneous detection of BRCA mutations and large genomic rearrangements in germline DNA and FFPE tumor samples. Oncotarget (2016) 7(38):61845–59. 10.18632/oncotarget.11259
18.
BozsikAPappJGrolmuszVKPatócsAOláhEButzH. Reclassification of five BRCA1/2 variants with unknown significance using complex functional study. Cancer Res Treat (2022) 54(4):970–84. 10.4143/crt.2021.1078
19.
JanavičiusR. Founder BRCA1/2 mutations in the Europe: implications for hereditary breast-ovarian cancer prevention and control. EPMA J (2010) 1(3):397–412. 10.1007/s13167-010-0037-y
20.
GradisharWJMoranMSAbrahamJAbramsonVAftRAgneseDet alBreast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2024). 22(5):331–357. 10.6004/jnccn.2024.0035
21.
DesaiSJenaAB. Do celebrity endorsements matter? Observational study of BRCA gene testing and mastectomy rates after angelina Jolie's New York times editorial. BMJ (2016) 355:i6357. 10.1136/bmj.i6357
22.
BedrosianISomerfieldMRAchatzMIBougheyJCCuriglianoGFriedmanSet alGermline testing in patients with breast cancer: ASCO-society of surgical oncology guideline. J Clin Oncol (2024) 42(5):584–604. 10.1200/JCO.23.02225
23.
ManahanERKuererHMSebastianMHughesKSBougheyJCEuhusDMet alConsensus guidelines on genetic` testing for hereditary breast cancer from the American society of breast surgeons. Ann Surg Oncol (2019) 26(10):3025–31. 10.1245/s10434-019-07549-8
24.
WhitworthPWBeitschPDPatelRRosenBCompagnoniGBaronPLet alClinical utility of universal germline genetic testing for patients with breast cancer. JAMA Netw Open (2022) 5(9):e2232787. 10.1001/jamanetworkopen.2022.32787
25.
KurianAWBernhiselRLarsonKCaswell-JinJLShadyabAHOchs-BalcomHet alPrevalence of pathogenic variants in cancer susceptibility genes among women with postmenopausal breast cancer. JAMA (2020) 323(10):995–7. 10.1001/jama.2020.0229
26.
YadavSHuCHartSNBoddickerNPolleyECNaJet alEvaluation of germline genetic testing criteria in a hospital-based series of women with breast cancer. J Clin Oncol (2020) 38(13):1409–18. 10.1200/JCO.19.02190
27.
LubinskiJKotsopoulosJMollerPPalTEisenAPeckLet alMRI surveillance and breast cancer mortality in women with BRCA1 and BRCA2 sequence variations. JAMA Oncol (2024) 10(4):493–9. 10.1001/jamaoncol.2023.6944
28.
LambertiniMBlondeauxETomaselloLMAgostinettoEHamyASKimHJet alClinical behavior of breast cancer in young BRCA carriers and prediagnostic awareness of germline BRCA status. J Clin Oncol (2025) 43(14):1706–19. 10.1200/JCO-24-01334
29.
GeyerCEJGarberJEGelberRDYothersGTaboadaMRossLet alOverall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol (2022) 33(12):1250–68. 10.1016/j.annonc.2022.09.159
30.
LittonJKHurvitzSAMinaLARugoHSLeeKHGonçalvesAet alTalazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol (2020) 31(11):1526–35. 10.1016/j.annonc.2020.08.2098
31.
ManchandaRPatelSGordeevVSAntoniouACSmithSLeeAet alCost-effectiveness of population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. J Natl Cancer Inst (2018) 110(7):714–25. 10.1093/jnci/djx265
32.
PatócsANagyPPappJBozsikAAntalBGrolmuszVKet alCost-effectiveness of genetic testing of endocrine tumor patients using a comprehensive hereditary cancer gene panel. J Clin Endocrinol Metab (2024) 109(12):3220–33. 10.1210/clinem/dgae300
33.
KurianAWWardKCAbrahamsePHamiltonASDeapenDMorrowMet alAssociation of germline genetic testing results with locoregional and systemic therapy in patients with breast cancer. JAMA Oncol (2020) 6(4):e196400. 10.1001/jamaoncol.2019.6400
34.
SunLBrentnallAPatelSBuistDSMBowlesEJAEvansDGRet alA cost-effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol (2019) 5(12):1718–30. 10.1001/jamaoncol.2019.3323
35.
GuoFAdekanmbiVHsuCDBerensonABKuoYFShihYT. Cost-effectiveness of population-based multigene testing for breast and ovarian cancer prevention. JAMA Netw Open (2024) 7(2):e2356078. 10.1001/jamanetworkopen.2023.56078
36.
VidraRCiuleanuTENemeşAPascuOHeroiuAMAntoneNet alSpectrum of BRCA1/2 mutations in Romanian breast and ovarian cancer patients. Int J Environ Res Public Health (2022) 19(7):4314. 10.3390/ijerph19074314
37.
AtcıMMGeredeliÇAySSakinAErtürkBSeçmelerŞet alClinical and pathological characteristics of patients with high-risk breast cancer based on BRCA mutation profiles: a retrospective study. Eur J Breast Health (2021) 17(2):123–7. 10.4274/ejbh.galenos.2020.6346
38.
NagyPPappJGrolmuszVKBozsikAPóczaTOláhEet alComprehensive clinical genetics, molecular and pathological evaluation efficiently assist diagnostics and therapy selection in breast cancer patients with hereditary genetic background. Int J Mol Sci (2024) 25(23):12546. 10.3390/ijms252312546
39.
PensabeneMVon ArxCDe LaurentiisM. Male breast cancer: from molecular genetics to clinical management. Cancers (Basel) (2022) 14(8):2006. 10.3390/cancers14082006
40.
SilvestriVBarrowdaleDMulliganAMNeuhausenSLFoxSKarlanBYet alMale breast cancer in BRCA1 and BRCA2 mutation carriers: pathology data from the consortium of investigators of modifiers of BRCA1/2. Breast Cancer Res (2016) 18(1):15. 10.1186/s13058-016-0671-y
41.
WongESShekarSChanCHHongLZPoonSYSillaTet alPredictive factors for BRCA1 and BRCA2 genetic testing in an Asian clinic-based population. PLoS One (2015) 10(7):e0134408. 10.1371/journal.pone.0134408
42.
KimEKParkSYKimSW. Clinicopathological characteristics of BRCA-Associated breast cancer in Asian patients. J Pathol Transl Med (2020) 54(4):265–75. 10.4132/jptm.2020.04.07
43.
HallMJReidJEBurbidgeLAPrussDDeffenbaughAMFryeCet alBRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer [published correction appears in cancer. 2009 Jun 15;115(12):2804]. Cancer (2009) 115(10):2222–33. 10.1002/cncr.24200
44.
de BockGHTollenaarRAPapelardHCornelisseCJDevileePvan de VijverMJ. Clinical and pathological features of BRCA1 associated carcinomas in a hospital-based sample of Dutch breast cancer patients. Br J Cancer (2001) 85(9):1347–50. 10.1054/bjoc.2001.2103
45.
Guzmán-ArochoYDRosenbergSMGarberJEVardehHPoorvuPDRuddyKJet alClinicopathological features and BRCA1 and BRCA2 mutation status in a prospective cohort of young women with breast cancer. Br J Cancer (2022) 126(2):302–9. 10.1038/s41416-021-01597-2
46.
VerhoogLCBrekelmansCTSeynaeveCvan den BoschLMDahmenGvan GeelANet alSurvival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet (1998) 351(9099):316–21. 10.1016/s0140-6736(97)07065-7
47.
ArmesJEEganAJSoutheyMCDiteGSMcCredieMRGilesGGet alThe histologic phenotypes of breast carcinoma occurring before age 40 years in women with and without BRCA1 or BRCA2 germline mutations: a population-based study. Cancer (1998) 83(11):2335–45.
48.
MusolinoABellaMABortesiBMichiaraMNaldiNZanelliPet alBRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study. Breast (2007) 16(3):280–92. 10.1016/j.breast.2006.12.003
49.
LangGTShiJXHuXZhangCHShanLSongCGet alThe spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer (2017) 141(1):129–42. 10.1002/ijc.30692
50.
FoulkesWDWongNBrunetJSBéginLRZhangJCMartinezJJet alGerm-line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin Cancer Res (1997) 3(12 Pt 1):2465–9.
51.
RobsonMGilewskiTHaasBLevinDBorgenPRajanPet alBRCA-associated breast cancer in young women. J Clin Oncol (1998) 16(5):1642–9. 10.1200/JCO.1998.16.5.1642
52.
MavaddatNBarrowdaleDAndrulisILDomchekSMEcclesDNevanlinnaHet alPathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the consortium of investigators of modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev (2012) 21(1):134–47. 10.1158/1055-9965.EPI-11-0775
53.
MarcusJNWatsonPPageDLNarodSALenoirGMToninPet alHereditary breast cancer: pathobiology, prognosis, and BRCA1 and BRCA2 gene linkage. Cancer (1996) 77(4):697–709. 10.1002/(sici)1097-0142(19960215)77:4<697::aid-cncr16>3.0.co;2-w
54.
YadavSHuCNathansonKLWeitzelJNGoldgarDEKraftPet alGermline pathogenic variants in cancer predisposition genes among women with invasive lobular carcinoma of the breast. J Clin Oncol (2021) 39(35):3918–26. 10.1200/JCO.21.00640
55.
AtchleyDPAlbarracinCTLopezAValeroVAmosCIGonzalez-AnguloAMet alClinical and pathologic characteristics of patients with BRCA-Positive and BRCA-negative breast cancer. J Clin Oncol (2008) 26(26):4282–8. 10.1200/JCO.2008.16.6231
56.
CopsonERMaishmanTCTapperWJCutressRIGreville-HeygateSAltmanDGet alGermline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol (2018) 19(2):169–80. 10.1016/S1470-2045(17)30891-4
57.
LakhaniSRVan De VijverMJJacquemierJAndersonTJOsinPPMcGuffogLet alThe pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol (2002) 20(9):2310–8. 10.1200/JCO.2002.09.023
58.
MalainouCPStachikaNDamianouAKAnastopoulosAPloumakiITriantafyllouEet alEstrogen-receptor-low-positive breast cancer: pathological and clinical perspectives. Curr Oncol (2023) 30(11):9734–45. 10.3390/curroncol30110706
Summary
Keywords
BRCA1/2, breast cancer, cancer susceptibility genes, germline testing, medical genetics
Citation
Nikolényi A, Dobi Á, Sántha D, Kószó R, Iványi M, Horváth E, Enyedi MZ, Priskin K, Csányi B, Patócs A, Butz H, Papp J, Varga Z, Tóth R, Oláh J and Kahán Z (2026) Germline BRCA testing in routine clinical practice: a single-center experience. Pathol. Oncol. Res. 32:1612238. doi: 10.3389/pore.2026.1612238
Received
05 August 2025
Revised
10 November 2025
Accepted
16 January 2026
Published
20 February 2026
Volume
32 - 2026
Edited by
Janina Kulka, Semmelweis University, Hungary
Updates
Copyright
© 2026 Nikolényi, Dobi, Sántha, Kószó, Iványi, Horváth, Enyedi, Priskin, Csányi, Patócs, Butz, Papp, Varga, Tóth, Oláh and Kahán.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zoltán Varga, varga.zoltan@med.u-szeged.hu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.