Abstract
Introduction:
Hairy cell leukemia (HCL) is an indolent B-cell lymphoproliferative disease. Interferon-alpha (IFN-alpha) was the first successfully used drug in HCL; its favourable effect has been known since the early 1980s. However, currently the first-line treatment of the disease consists of purine nucleoside analogs.
Objectives:
The aim of our study was to assess the efficacy of pegylated IFN-alpha in HCL patients treated with this drug at a single university center.
Methods:
We report the treatment characteristics and outcome of seven classical HCL patients treated with pegylated IFN-alpha at the Department of Internal Medicine and Oncology, Semmelweis University.
Results:
As a result of pegylated interferon-alpha treatment, 3 of 7 patients (3/7) achieved an unconfirmed complete remission, 3 of 7 patients (3/7) achieved partial remission. One patient had stable disease while receiving pegylated IFN-alpha. Only mild adverse effects and no infectious complications were observed during our treatment.
Conclusion:
Our clinical data support that pegylated IFN-alpha in monotherapy is effective and safe even in elderly and frail HCL patients. It may also be a preferred therapeutic option in patients with profound immunosuppression and in patients with severe active infections.
Introduction
Hairy cell leukemia (HCL) is an indolent B-cell lymphoproliferative disease characterized by pancytopenia, splenomegaly, and infiltration of bone marrow, liver, and spleen with mature B cells. HCL is a rare neoplasm representing 2% of lymphomas [1, 2]. The disease is well characterized based on cytomorphologic and immunophenotypic features, and its defining parameters as a distinct entity have remained unchanged since the 2001 WHO classification [3].
Common symptoms arise as a consequence of cytopaenias and splenomegaly [4]. Infectious complications are common, due to both the underlying immunosuppression from granulocytopenia and monocytopenia and decreased activity of NK cells and dendritic cells [3].
The diagnosis is based on the histological examination of the bone marrow trephine biopsy specimen [5]. The well-established surface antigen expression pattern (CD20+ CD103+ CD25+) were complemented by a genetic marker in 2011 thanks to the groundbreaking discovery of the p.V600E mutation in the BRAF gene characteristic of this entity [6].
The current gold standard first-line treatment of hairy cell leukemia is the administration of purine nucleoside analogs (cladribine or pentostatin) [7]. Purine nucleoside analogues are highly immunosuppressive, thus ongoing infections in HCL patients limit their usability [8]. Furthermore, during the COVID-19 pandemic BRAF inhibitors came into play as an alternative therapy since they do not cause neutropaenia [9]. Since this indication is off-label for BRAF inhibitors and they are not available worldwide, the search for a new alternative has begun. Recombinant Interferon-alpha is recommended as a first-line treatment in pregnancy, in patients with severe neutropenia or in those who are not eligible for purine nucleoside analogues [10]. At the end of 2019 the production of both types of recombinant interferon-alpha was discontinued worldwide, which coincided with the COVID-19 pandemic and created a crisis situation in this regard. Thus, the administration of pegylated interferon-alpha (peginterferon alfa-2a) (PEG-IFN) became available in Hungary as a possible alternative.
Patients and methods
During our analysis we complied with the Helsinki declaration and its later amendments. All participants gave their informed consent to the analysis of the data. The Medical Research Council has granted the study its approval under the ethical number 28564-2/2017/EKU.
A total of 7 HCL patients with classical phenotype (4 males, 3 females) received pegylated interferon-alpha treatment at the Department of Internal Medicine and Oncology, Semmelweis University. These patients have been treated previously (between 2016 and 2020) with recombinant interferon-alpha, except for 3 patients who received pegylated interferon-alpha as a first line treatment. Pegylated interferon-alpha became available from the early months of 2020. Our follow-up period ended on October 31st of 2024. The median age at diagnosis was 67 years (range: 48–73 years, mean: 63.71 years), and at initiation of pegylated interferon-alpha treatment was 69,5 years (range: 59–75 years, mean: 69.14 years). The median time from diagnosis to PEG-IFN treatment was 24 months (range, 1–168 months). Patient characteristics are shown in Table 1.
TABLE 1
| Patient | Year of birth | Sex | Age at HCL diagnosis | Comorbidities |
|---|---|---|---|---|
| Patient 1 | 1961 | male | 48 | hypertension, mesenteric thrombosis, type 2 diabetes mellitus, umbilical hernia |
| Patient 2 | 1950 | female | 63 | hiatus hernia, gonarthrosis, hypertension, thyreoiditis, postmenopausal osteoporosis |
| Patient 3 | 1946 | female | 72 | liver cysts, uterine myoma, hypertension, angina pectoris |
| Patient 4 | 1949 | male | 71 | nephrolithiasis, hypertension, hypercholesterolaemia, type 2 diabetes mellitus, GERD, hiatus hernia, basal-cell carcinoma, benign prostate hyperplasia, |
| Patient 5 | 1952 | male | 55 | tonsillectomy, type 2 diabetes mellitus, gonarthrosis, chronic cholecystitis, hypertension, dilatative cardiomyopathy, amputation of both legs below the knee due to diabetes complications |
| Patient 6 | 1957 | female | 64 | insulin resistance |
| Patient 7 | 1947 | male | 73 | nephrolithiasis, lumboischialgia, prostate cancer, glaucoma, coxarthrosis, rectal cancer |
Patient characteristics of our cohort.
Our diagnostic workup consisted of taking a detailed past medical history, a thorough physical examination, peripheral blood counts and flow cytometric evaluations according to standard procedures. The ultimate diagnostic procedure was the histological examination of the bone marrow biopsy sample. BRAF status was determined by immunohistochemistry, while the sample of one of the patient a had been studied by polymerase chain reaction and pyrosequencing of the product and in the this patient. In the later course of disease, BRAF positivity was proven by immunohistochemistry. Detailed description of BRAF V600E mutation–specific immunohistochemistry and the molecular analysis for BRAF mutations can be found in our 2023 publication [9].
When suspecting a relapse, we performed these examinations including BRAF immunohistochemistry again to confirm the relapse of the hairy cell leukaemia.
Clinical data were collected at regular follow-up visits and by medical chart review. Responses were evaluated based on standard criteria as published by Bohn et al [11]. A response was categorized as an unconfirmed complete remission (CRu) if the blood counts normalized and organomegaly resolved but no bone marrow biopsy was carried out to confirm the complete remission. Partial remission (PR) was defined as more than 50% improvement in cell counts, shrinking organ sizes and an at least 50% decrease in bone marrow infiltration as judged by the pathologist. Stable disease was defined when no significant changes in blood counts or organomegaly were observed [11].
Results
At our clinic we have treated seven classical HCL patients with pegylated interferon-alpha between July of 2016 and October 31st of 2024 (interferon-alpha treatment until early months of 2020 and pegylated interferon-alpha from then on). Our mean follow-up time from first referral to our center was 92.14 months (range: 32–208 months). As previous treatment lines, one patient was heavily pretreated, receiving PEG-IFN-alpha in 6th line, one further patient received PEG-IFN-alpha in 4th line. In three patients pegylated IFN-alpha was administered as first-line treatment. In these patients excessive bone marrow infiltration was present. In 3 patients the pegylated IFN-alpha treatment was still ongoing at the time of analysis. Results of the pegylated IFN-alpha treatment in hairy cell leukaemia patients can be seen in Table 2.
TABLE 2
| Patient | Therapy start | Dose | Previous therapy lines | Response to therapy | Adverse events | Therapy discontinuation | Duration of PEG-IFN-alpha treatment | Duration of PEG-IFN-alpha treatment response | Current therapy | Duration of follow-up from first referral to the center |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 2016.07.15 (interferon alpha), 2020.08.24 (pegylated IFN-alpha) | 90 µg weekly | 1st line: interferon alpha for several months, 2nd line: cladribine, 3rd line: one time rituximab infusion, 4th line: vemurafenib, 5th line: interferon-alpha, 6th line: PEG-IFN-alpha | CRu | liver enzyme elevation, nausea | due to liver enzyme elevation in the fall of 2022 | 26 months | 11 months | venetoclax 400 mg/die | 178 months |
| Patient 2 | 2022.02.22 | 45 µg weekly | 1st line: PEG-IFN-alpha | PR | none reported | April of 2022, due to next treatment line initiation (cladribine) | 2 months | 1,8 months | no hematological therapy needed | 32 months |
| Patient 3 | 2018.08.22 (interferon alpha), 2021.07.16 (pegylated IFN-alpha) | 45 µg weekly | 1st line: interferon alpha, 2nd line: PEG-IFN-alpha | CRu | none reported | ongoing pegylated IFN-alpha therapy | 39 months | 39 months | ongoing pegylated IFN-alpha therapy | 74 months |
| Patient 4 | 2020.01.04 (interferon alpha), 2020.04.05 (pegylated IFN-alpha) | 90 µg weekly and from February of 2022 45 µg weekly | 1st line: interferon alpha, 2nd line: PEG-IFN-alpha | CRu | none reported | March of 2023, due to complete remission | 35 months | 54 months | no hematological therapy needed | 57 months |
| Patient 5 | 2007-2010 (interferon alpha), 2020.12.23 (pegylated IFN-alpha) | 90 µg weekly and from February of 2023 45 µg weekly | 1st line: interferon alpha, 2nd line: cladribine, 3rd line: rituximab infusions 4 times, 4th line: PEG-IFN-alpha | PR | none reported | ongoing pegylated IFN-alpha therapy | 47 months | 47 months | ongoing pegylated IFN-alpha therapy | 208 months |
| Patient 6 | 2021.02.04 (pegylated IFN-alpha) | 45 µg weekly due to severe granulopaenia, then 90 µg weekly from 12th of April 2021 on | 1st line: PEG-IFN-alpha | stable disease | flatulence | in July of 2021 due to next treatment line initiation (cladribine) | 5 months | 4,8 months | no hematological therapy needed | 46 months |
| Patient 7 | 2021.01.14 (pegylated IFN-alpha) | 90 µg weekly | 1st line: PEG-IFN-alpha | PR | none reported | ongoing pegylated IFN-alpha therapy | 45 months | 45 months | ongoing pegylated IFN-alpha therapy | 50 months |
Results and characteristics of the pegylated IFN-alpha treatment in hairy cell leukaemia patients.
CRu, unconfirmed complete remission. PR, partial remission.
We administered IFN-alpha in pegylated formulation weekly (45–90 µg/week) by subcutaneous injection. As a result of treatment with pegylated interferon-alpha, 3 of 7 patients (3/7) achieved an unconfirmed complete remission, 3 of 7 patients (3/7) achieved partial remission. One patient had stable disease while receiving pegylated interferon-alpha. The detailed absolute neutrophil count (ANC) and platelet counts (PLT) can be seen in the Supplementary Material for each patient. An example is shown in Figure 1 for the case of patient 4.
FIGURE 1
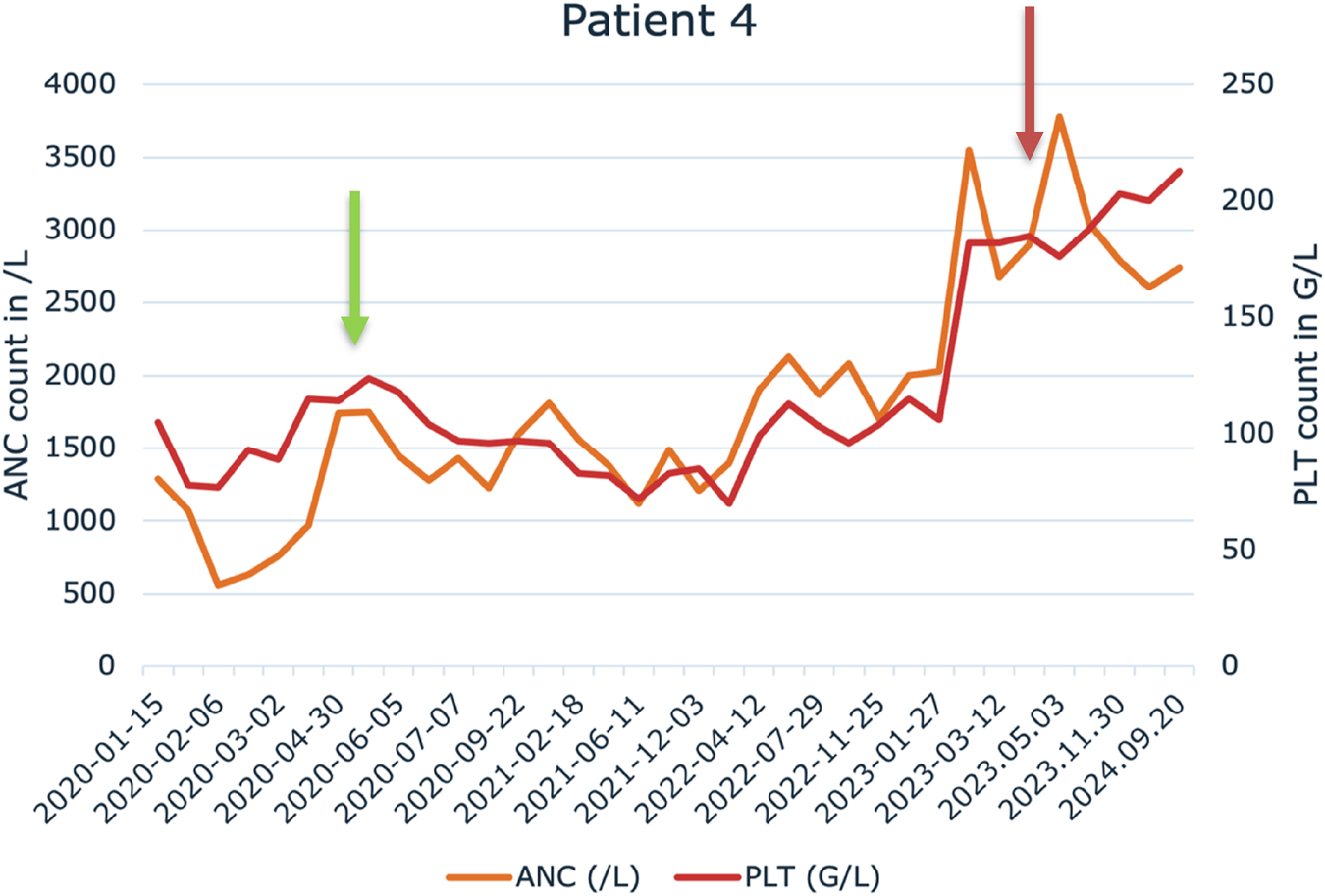
Change in absolute neutrophile count (ANC) and platelet (PLT) levels during the pegylated IFN-alpha treatment in Patient 4. Green arrow indicates the start of the treatment and red arrow indicates the cessation of the pegylated IFN-alpha therapy.
No drug-related reactions or infectious complications were observed during our treatment. Three patients who received pegylated IFN-alpha once a week on a continous basis achieved partial remission. In the case of two patients who received cladribine treatment after pegylated IFN-alpha, after the completion of cladribine treatment hairy cells were not detected in the bone marrow anymore. During this follow-up time all patients are still alive. During our follow-up of Patient 7, we encountered a p16 positive squamous cell carcinoma of the anal canal 28 months after pegylated interferon-alpha treatment has started. In his past medical history a prostate adenocarcinoma is known, he underwent surgery for that in 2014, 7 years prior to starting PEG-IFN therapy.
Discussion
The current gold standard first-line treatment of hairy cell leukemia is the administration of purine nucleoside analogs (PNA), such as cladribine or pentostatin [7]. Thanks to PNA treatment, the life expectancy of patients today does not differ from the life expectancy of the average healthy population, but 40% still have a relapse [12, 13]. While purine analogues are effective and safe in middle aged or younger and fit patients, in the treatment of older and unfit patients PNAs yield an unsatisfactory response [10].
The clinical activity of IFN-alpha in HCL patients has been known since the early 1980s. IFN induced responses were partial and of relatively short duration and most patients eventually relapsed after discontinuing treatment, with a median time to treatment failure ranging from 6 to 25 months [14]. It has been also reported, that long term maintenance IFN-alpha therapy after a short induction period can lead to longer remission periods and durable response [15, 16]. Also, in treatment naïve patients minimal doses of IFN-alpha seem to be effective [17]. In our cohort we did not observe a serious side effect leading to treatment discontinuation, which also fits the literature data [18].
Our results are in good concordance with the literature data. A 30 years retrospective analysis from Italy has found that IFN-alpha treatment of 74 HCL patients yielded a favorable response in almost all patients and even CR was achieved in 24% of patients [19]. This comprehensive study also highlights 3 key areas of use for IFN-alpha: patients with comorbidities that limit therapeutic agents, elderly patients, and times of a serious pandemic (due to its immunomodulant rather than immunosuppressive properties) [19–21]. Also, as we have seen in over the course of the COVID-19, that IFN-alpha is suggested by international consensus guidelines over PNAs, since it does not cause a drop in neutrophil counts thus does not elevate the risk of infection in these patients [20].
In the 1990-s the occurrence of secondary neoplasms in HCL patients treated with IFN have been first described. Contradicting reports have been published on the topic, an Italian group did not find an elevated risk of secondary neoplasms among HCl patients, while in a large cohort an increased incidence of secondary tumors, especially Hodgkin and non-Hodgkin lymphomas was observed [22, 23]. Our group also reported on the development of diffuse large B-cell lymphoma in two HCL patients a long time after their HCL diagnosis [24]. Our results from our small patient cohort do not allow to draw any conclusion in this respect.
Dosing of pegylated interferon-alpha treatment in our opinion should start with 45 µg weekly since neutrophil numbers may still be dropping at the dose of 45 µg weekly (as seen in the supplementary figure in case of Patient 2), which is crucial in an ongoing infection or in a severely immunocompromised state. We hypothesize that by administering a dose of 90 µg weekly the granulocyte drop would be even more pronounced. We advise starting the treatment with 45 µg weekly and slowly titrating upward to the effective dose (usually 90 µg weekly).
Only two case reports have been published until now showing the efficacy of PEG-IFN-alpha HCL. In April 2022 Furlan et al., reported on a 73-year-old maleimmunosuppressed HCL patient with Mycobacterium subsp. massilliense abscess. Due to the ongoing infection and his severely immunocompromised status his HCL disease was treated with a combination of vemurafenib and rituximab after a “priming” phase with PEG-IFN-alpha as a first-line treatment (starting dose of 135 µg weekly). However, vemurafenib had to be discontinued after only 4 days of treatment due to QT interval prolongation and the possibility of cardiac toxicity. After the discontinuation of vemurafenib, PEG IFNα-2a was administered alongside rituximab again until the resolution of the infection. Two months after the first rituximab infusion a control bone marrow trephine biopsy was carried out which proved a complete response to the treatment [25]. This treatment combination might be a potential therapeutic option in patients with active severe infection and for whom the use of purine nucleoside analogues (PNA) is contraindicated [25]. In December 2022 De Novellis et al., reported an old and frail HCL patient treated with pegylated IFN-alpha in monotherapy (90 µg once a week) as first-line treatment. The patient had severe neutropenia. After 6 months of therapy the patient achieved complete remission [26].
IFN-alpha binds to cell surface receptors with two particular subunits: IFN-α receptor-1 and IFN-α receptor-2 and activates the JAK/STAT signaling pathways and leads to the expression of antiproliferative and proapoptotic genes [27–29]. Moreover, it can also inhibit hematopoietic stem cell growth [30]. These effects reduce leukemic cell growth and cause myelosuppression [27, 28, 30]. The administration of IFN-alpha in pegylated form before and during rituximab treatment could be effective because IFN-alpha can increase CD20 antigen expression on hairy cells and simultaneously stimulate CD8 T cell and NK cytotoxic activity and induce neoplastic clone suppression [25].
Our data also support that pegylated IFN-alpha monotherapy is effective and safe in old and frail HCL patients. It can also be a preferred therapeutic option in patients with profound immunosuppression and in patients with severe active infections. In the treatment course we suggest monitoring the complete blood counts, liver function tests and clinical chemistry values.
PEG-IFN is not recommended for patients with autoimmune disorders, as the development of autoantibodies and autoimmune disorders has been reported during treatment with alfa interferons, according to the summary of product characteristics. Patients predisposed to the development of autoimmune disorders may be at increased risk. Patients with signs or symptoms consistent with autoimmune disorders should be evaluated carefully, and the benefit-risk of continued interferon therapy should be re-assessed (accessed on the 3rd of January 20251). Premedication (e.g., acetaminophen) prior to pegylated IFN-alpha treatment may reduce flu-like effects.
Conclusion
In our current work, we present data and clinical outcomes of 7 patients diagnosed with hairy cell leukaemia who received pegylated interferon-alpha treatment at our tertiary center between 2016 and 2024. Our analysis shows that pegylated interferon-alpha is a safe and effective treatment even in elderly and frail HCL patients who suffer from severe cytopaenias and/or from ongoing infections. Our relatively long follow-up confirmed the efficacy of this treatment. Moreover, 3 of the patients are in an unconfirmed complete remission with no current need for hematological therapy, 3 more patients achieved partial remission and are on long-term pegylated interferon-alpha injections without notable side effects.
Overall, we recommend that the administration of pegylated interferon-alpha be considered in selected cases of hairy cell leukaemia either as first line treatment or in further lines, depending on the clinical situation.
Statements
Data availability statement
The datasets presented in this article are not readily available because data was analyzed from medical files which contain private information.
Ethics statement
The Medical Research Council has granted the study its approval under the ethical number 28564-2/2017/EKU. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: KF and JD. Methodology: KF, BT, and JD. Patient care: KF, ZN, II, HE, JD. Formal analysis: KF, ZN, and BT. Investigation: KF, ZN, II, HE, CB, BT, and JD. Resources: CB and JD. Writing – original draft: KF, ZN, and JD. Writing – review and editing: ZN, BT, and JD. Supervision: BT and JD. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank the patients for participating in this study, Anita Deli for her skilled technical assistance and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2025.1612108/full#supplementary-material
Footnotes
1.^ https://www.ema.europa.eu/en/documents/product-information/pegasys-epar-product-information_en.pdf
References
1.
Sári E Nagy Z Demeter J . Visszatérő szomatikus mutáció hajas sejtes leukémiában [Recurrent somatic mutation in hairy cell leukemia]. Orv Hetil (2013) 154:123–7. 10.1556/OH.2013.29531
2.
Troussard X Maître E Paillassa J . Hairy cell leukemia 2024: update on diagnosis, risk-stratification, and treatment—annual updates in hematological malignancies. Am J Hematol (2024) 99:679–96. 10.1002/ajh.27240
3.
Grever MR Abdel-Wahab O Andritsos LA Banerji V Barrientos J Blachly JS et al Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood (2017) 129:553–60. 10.1182/blood-2016-01-689422
4.
Cross M Dearden C . Hairy cell leukaemia. Curr Oncol Rep (2020) 22:42. 10.1007/s11912-020-00911-0
5.
Troussard X Maître E Cornet E . Hairy cell leukemia 2022: update on diagnosis, risk-stratification, and treatment. Am J Hematol (2022) 97:226–36. 10.1002/ajh.26390
6.
Tiacci E Trifonov V Schiavoni G Holmes A Kern W Martelli MP et al BRAF mutations in hairy-cell leukemia. N Engl J Med. (2011) 364:2305–15. 10.1056/NEJMoa1014209.BRAF
7.
Maitre E Cornet E Troussard X . Hairy cell leukemia: 2020 update on diagnosis, risk stratification, and treatment. Am J Hematol (2019) 94:1413–22. 10.1002/ajh.25653
8.
Bohn JP Dietrich S . Treatment of classic hairy cell leukemia: targeting minimal residual disease beyond cladribine. Cancers (Basel) (2022) 14:956–13. 10.3390/cancers14040956
9.
Ferenczi K Nagy ZF Istenes I Eid H Bödör C Timár B et al Long term follow-up of refractory/relapsed hairy cell leukaemia patients treated with low-dose vemurafenib between 2013 and 2022 at the Department of Internal Medicine and Oncology, Semmelweis University. Semmelweis Univ Pathol Oncol Res (2023) 29:1611378. 10.3389/pore.2023.1611378
10.
Robak T Matutes E Catovsky D Zinzani PL Buske C , ESMO Guidelines Committee. Hairy cell leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26:v100–v107. 10.1093/annonc/mdv200
11.
Bohn JP Neururer S Pirklbauer M Pircher A Wolf D . Hairy cell leukemia patients have a normal life expectancy—a 35-year single-center experience and comparison with the general population. Cancers (Basel) (2022) 14:1242. 10.3390/cancers14051242
12.
Saven A Burian C Koziol JA Piro LD . Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood (1998) 92:1918–26. 10.1182/blood.v92.6.1918
13.
Dinmohamed AG Posthuma EFM Visser O Kater AP Raymakers RAP Doorduijn JK . Relative survival reaches a plateau in hairy cell leukemia: a population-based analysis in The Netherlands. Blood (2018) 131:1380–3. 10.1182/blood-2017-12-820381
14.
Ahmed S Rai KR . Interferon in the treatment of hairy-cell leukemia. Best Pract Res Clin Haematol (2003) 16:69–81. 10.1016/S1521-6926(02)00084-1
15.
Benz R Siciliano RD Stussi G Fehr J . Long-term follow-up of interferon-alpha induction and low-dose maintenance therapy in hairy cell leukemia. Eur J Haematol (2009) 82:194–200. 10.1111/j.1600-0609.2008.01190.x
16.
Damasio EE Clavio M Masoudi B Isaza A Spriano M Rossi E et al Alpha-interferon as induction and maintenance therapy in hairy cell leukemia: a long-term follow-up analysis. Eur J Haematol (2000) 64:47–52. 10.1034/j.1600-0609.2000.90014.x
17.
Gastl G Aulitzky W Tilg H Thaler J Berger M Huber C . Minimal interferon-alpha doses for hairy cell leukemia. Blood (1990) 75:812–3. 10.1182/blood.v75.3.812.bloodjournal753812
18.
Bohn JP Gastl G Steurer M . Long-term treatment of hairy cell leukemia with interferon-α: still a viable therapeutic option. Memo - Mag Eur Med Oncol (2016) 9:63–5. 10.1007/s12254-016-0269-1
19.
Assanto GM Riemma C Malaspina F Perrone S De Luca ML Pucciarini A et al The current role of interferon in hairy cell leukaemia: clinical and molecular aspects. Br J Haematol (2021) 194:78–82. 10.1111/bjh.17440
20.
Grever M Andritsos L Banerji V Barrientos JC Bhat S Blachly JS et al Hairy cell leukemia and COVID-19 adaptation of treatment guidelines. Leukemia (2021) 35:1864–72. 10.1038/s41375-021-01257-7
21.
Wierda WG Byrd JC Abramson JS Bhat S Bociek G Brander D et al Hairy cell Leukemia, Version 2.2018: clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw (2017) 15:1414–27. 10.6004/jnccn.2017.0165
22.
Hisada M Chen BE Jaffe ES Travis LB . Second cancer incidence and cause-Specific mortality among 3104 patients with hairy cell leukemia: a population-based study. J Natl Cancer Inst (2007) 99:215–22. 10.1093/jnci/djk030
23.
Federico M Luigi Zinzani P Frassoldati A Vinceti M Modè A Annino L et al Risk of second cancer in patients with hairy cell leukemia: long-term follow-up. J Clin Oncol (2002) 20:638–46. 10.1200/JCO.2002.20.3.638
24.
Nagy ZF Ferenczi K Istenes I Eid H Bödör C Timár B et al Case report: development of diffuse large B cell lymphoma a long time after hairy cell leukemia. Pathol Oncol Res (2022) 28:1610338–5. 10.3389/pore.2022.1610338
25.
Furlan A Rossi MC Gherlinzoni F Scotton P . Prompt hematological recovery in response to a combination of pegylated interferon α-2a and rituximab in a profoundly immuno-suppressed hairy cell leukemia patient with a mycobacterial infection at onset: benefits and drawbacks of rapid immune reconstitution. Hematol Rep (2022) 14:135–42. 10.3390/hematolrep14020020
26.
De Novellis D Giudice V Ciccone V Erra P De Vita A Picone F et al A frail hairy cell leukemia patient successfully treated with pegylated interferon-α-2A. J Clin Med (2023) 12:193–11. 10.3390/jcm12010193
27.
Kiladjian JJ Mesa RA Hoffman R . The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood (2011) 117:4706–15. 10.1182/blood-2010-08-258772
28.
Healy FM Dahal LN Jones JRE Floisand Y Woolley JF . Recent progress in interferon therapy for myeloid malignancies. Front Oncol (2021) 11:769628–12. 10.3389/fonc.2021.769628
29.
Brierley MM Fish EN . IFN-α/β receptor interactions to biologic outcomes: understanding the circuitry. J Interf Cytokine Res (2002) 22:835–45. 10.1089/107999002760274845
30.
Chawla-Sarkar M Lindner DJ Liu YF Williams BR Sen GC Silverman RH et al Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis (2003) 8:237–49. 10.1023/A:1023668705040
Summary
Keywords
hairy cell leukemia, interferon, HCL, pegylated interferon-alpha, severe infection
Citation
Ferenczi K, Nagy ZF, Istenes I, Eid H, Bödör C, Timár B and Demeter J (2025) Successful treatment of hairy cell leukaemia with pegylated interferon-alpha-2A. Pathol. Oncol. Res. 31:1612108. doi: 10.3389/pore.2025.1612108
Received
13 February 2025
Accepted
07 May 2025
Published
21 May 2025
Volume
31 - 2025
Edited by
Hajnalka Rajnai, Semmelweis University, Hungary
Updates
Copyright
© 2025 Ferenczi, Nagy, Istenes, Eid, Bödör, Timár and Demeter.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zsófia Flóra Nagy, nagy.zsofia.flora@semmelweis.hu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.