Abstract
Objective:
We aimed to analyze the relationship between the quantitative and qualitative parameters of three-dimensional computed tomography (CT), epidermal growth factor receptor (EGFR), and anaplastic lymphoma kinase (ALK) in ground-glass opacity (GGO)-associated lung adenocarcinoma and determine their prognostic value.
Methods:
In total, 208 patients with GGO-associated lung adenocarcinoma admitted to our hospital from January 2019 to September 2021 were selected as study subjects. All participants underwent EGFR gene mutation and ALK gene rearrangement tests. The quantitative and qualitative parameters of three-dimensional CT scans were compared among patients with different EGFR gene mutations and ALK gene rearrangements. Multivariate analysis was conducted to investigate the association of these parameters with EGFR gene mutation and ALK gene rearrangement in patients with GGO-associated lung adenocarcinoma. Furthermore, the quantitative and qualitative parameters of three-dimensional CT scans were compared among patients with different prognoses, and the value of these parameters in predicting patients’ prognoses was analyzed.
Results:
There were significant differences between patients with wild-type EGFR and patients with mutant EGFR in terms of the bronchial sign (BS), pleural indentation sign (PIS), vascular bundle sign (VBS), maximum nodule diameter (MND), nodule volume (NV), average CT value (ACTV), and solid compartment proportion (SCP) (P < 0.05). There were significant differences between patients with and without ALK gene rearrangement in terms of the BS, PIS, VBS, ACTV, and SCP (P < 0.05). There was a significant difference in BS, PIS, VBS, MND, NV, ACTV, and solidity between patients with favorable prognosis and those with poor prognosis (P < 0.05). The AUC of the combination of BS, PIS, VBS, MND, NV, ACTV, and SCP for predicting patients’ prognosis was the highest, significantly higher than the AUC value of individual parameters (P < 0.05).
Conclusion:
The quantitative and qualitative parameters of three-dimensional CT are closely associated with EGFR gene mutations, ALK gene rearrangements, and prognosis in patients with GGO-associated lung adenocarcinoma. Moreover, each parameter holds a high value in predicting the prognosis of patients with GGO-associated lung adenocarcinoma.
Introduction
Based on recent reports, the incidence and mortality rates of lung cancer in China rank first among all malignant tumors, accounting for 22.0% of all new cancer cases and 28.5% of cancer-related deaths [1]. Lung adenocarcinoma is a common pathological type of lung cancer. Most patients with lung adenocarcinoma often have no obvious clinical symptoms in the early stages, and the cancer usually manifests as pulmonary nodules. With the increasing popularity of imaging technologies and growing awareness of physical examinations in recent years, the detection rate of ground-glass opacity (GGO)-associated lung adenocarcinoma has significantly increased [2]. GGO, an imaging manifestation on chest computed tomography (CT), appears as focal, cloudy, and hazy pale shadows with a higher density than normal lung tissue. CT is widely used in lung cancer screening, thereby improving the detection rate of GGO. Among lung nodules, there are many partial solid nodules with characteristics intermediate between those of pure ground-glass and solid nodules. Pure ground-glass and solid nodules may share the same imaging features, which can affect the interpretation of results [3]. With the continuous advancement of CT technology, three-dimensional CT scans can reconstruct three-dimensional stereoscopic lesions from multiple angles and directions, enabling the visualization of lesion characteristics in three dimensions and offering high value in the differential diagnosis of benign and malignant lung lesions, including GGO [4].
Epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements are common genetic alterations in lung adenocarcinoma. Lung biopsy is an invasive procedure that may increase the risk of pneumothorax, hemorrhage, and vascular injury [5]. Previous studies have primarily explored the relationship between CT imaging features, EGFR mutations, and ALK rearrangements in patients with lung cancer, often focusing separately on qualitative or quantitative parameters [6, 7]. However, few studies have comprehensively analyzed both qualitative and quantitative parameters of three-dimensional CT in relation to these genetic alterations and evaluated their prognostic significance. Therefore, this study aimed to provide a reference for understanding the imaging characteristics of lung adenocarcinoma associated with GGO.
Subjects and methods
Subjects
In total, 208 patients with GGO-associated lung adenocarcinoma admitted to our hospital from January 2019 to September 2021 were included as study subjects, including 139 males and 69 females. They were aged 46–72 years (59.41 ± 6.35 years). The tumor was on the left side in 118 cases and on the right side in 90 cases. Regarding clinical stage, 83 patients were in stage I and 125 patients were in stage IIA. Regarding pathological types, there were 79 cases of invasive adenocarcinoma, 86 cases of minimally invasive adenocarcinoma, 27 cases of adenocarcinoma in situ, and 16 cases of atypical adenomatous hyperplasia.
The inclusion criteria were as follows: 1) lung adenocarcinoma meeting the diagnostic criteria of the Chinese Standard for the Diagnosis and Treatment of Primary Lung Cancer (2015 Edition) [8]; 2) GGO-associated lung adenocarcinoma; 3) patient undergoing EGFR and ALK gene rearrangement testing; and 4) good quality of three-dimensional CT images. The exclusion criteria were as follows: 1) coexistence of other malignant tumors and other types of lung cancer; 2) undergoing surgery, radiotherapy, or chemotherapy 3 months before enrollment; 3) history of previous lung surgery; 4) hematologic diseases; or 5) mental or intellectual disabilities.
Methods
Three-dimensional CT examination
A Philips iCT 256-slice CT scanner was used for lung examination. The patients were in the supine position and instructed to hold their breath after deep inhalation. Scanning was conducted from the lung tip to the posterior costodiaphragmatic angle. Tube voltage, pitch, layer thickness, and matrix were 120 kV, 0.6 mm, 5 mm, and 512 × 512, respectively. For the lung window, we adopted a window width of 1500 Hu and a window position of 400 Hu. For the mediastinal window, the window width was 400 Hu and the window position was 40 Hu. The acquired two-dimensional images were transmitted to the workstation. The area of interest of the lesion was delineated, and the three-dimensional images were reconstructed. Two senior radiologists analyzed the three-dimensional images and obtained quantitative parameters, including the maximum nodule diameter (MND) and nodules volume (NV), average CT value (ACTV), and solid compartment proportion (SCP). Multi-plane reconstruction, maximum intensity projection, and other techniques were employed to observe the morphology of GGO. Qualitative indicators were obtained, including lesion location, nodule type, tumor shape, lobulation sign, cavitation, burr sign, vacuolar sign, bronchial sign (BS), pleural indentation sign (PIS), and vascular bundle sign (VBS).
Detection of EGFR and ALK gene rearrangement
Peripheral blood samples (4–5 mL) were collected from patients, and DNA was extracted using the QIAamp DNA Kit (Qiagen, Cat. No. 51183). DNA concentration was determined, and A260/280 was qualified in the range of 1.8∼2.0. The qualitative EGFR mutation detection kit and ALK fusion gene qualitative mutation detection kit (both from Xiamen AmoyDx Biotechnology Co., Ltd.; No. ADx-EG0X, ADx-AE01) were used following the manufacturers’ instructions to prepare the reaction system. PCR amplification was conducted using an ABI 7500 instrument under the following conditions: initial denaturation at 95°C for 5 min, followed by 15 cycles of 95°C for 25 s, 64°C for 20 s, and 72°C for 20 s; then 31 cycles of 93°C for 25 s, 60°C for 35 s, and 72°C for 20 s. EGFR mutations and ALK gene rearrangements were detected using the ARMS-PCR method. EGFR mutations were determined by comparing the sample Ct value with the threshold (ΔCt = sample Ct - quality control Ct). A Ct value <26 was regarded as a strong positive mutation. If 26≤Ct<29 and any of the following criteria were met the sample was classified as mutation-positive: ΔCt <11 for 19Del (exon 19), <11 for L858R (exon 21), <7 for T790M (exon 20), <9 for 20Ins (exon 20), <7 for G719X (exon 18), <8 for S768I (exon 20), or <8 for L861Q (exon 21). A Ct value of <26 was considered positive for ALK gene rearrangement. The primers used in this study were all designed and synthesized by the Beijing Genomics Institute (BGI). The representative qPCR amplification curves are shown in Figure 1. The primer sequences are shown in Table 1.
FIGURE 1
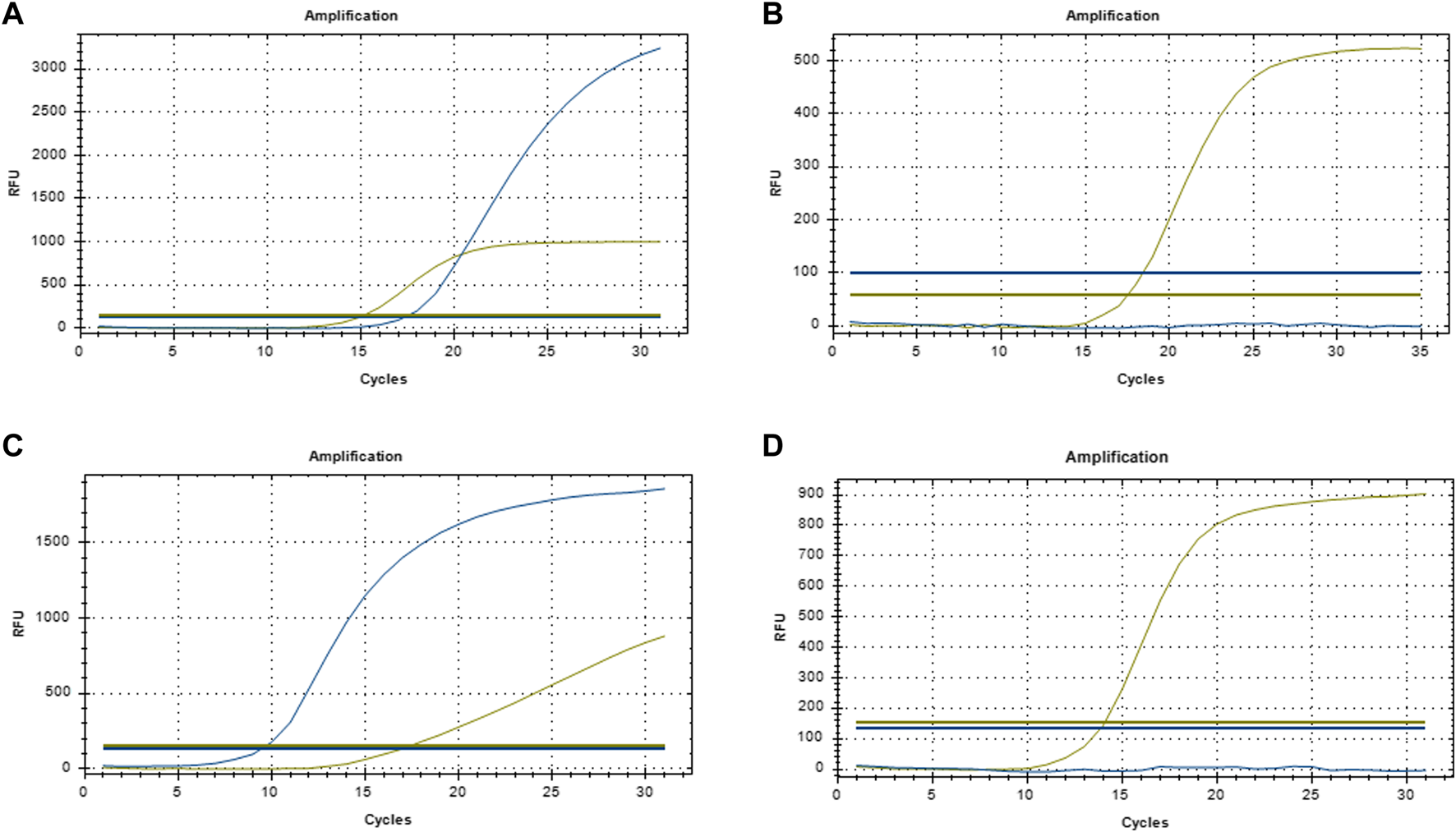
Representative qPCR amplification curve (A) EGFR-positive; (B) EGFR-negative; (C) ALK-positive; (D) ALK-negative.
TABLE 1
| Title | Primer |
|---|---|
| 19Del | Forward: 5′-GCACCATCTCACAATTGCCAGTTA-3′ |
| Reverse: 5′-ATGTGGAGATGAGCAGGGTCT-3′ | |
| T790M | Forward: 5′-CCATGAGTACGTATTTTGAAACTC-3′ |
| Reverse: 5′-CATATCCCCATGGCAAACTCTTGC-3′ | |
| L858R | Forward: 5′-CCTCACAGCAGGGTCTTCTCTGT-3′ |
| Reverse: 5′-TCCCTGGTGTCAGGAAAATGCT-3′ | |
| 20Ins | Forward: 5′-GAGGCACCCAGCACCTTCTT-3′ |
| Reverse: 5′-CAGGGTCTGGAGCAAATGCT-3′ | |
| G719X | Forward: 5′-CTTGTCGCTGGACATACTGG-3′ |
| Reverse: 5′-CCTCCTTACTTTGCCTCCTTCC-3′ | |
| S768I | Forward: 5′-GGACGTACTGGTGAAAACACC-3′ |
| Reverse: 5′-CAGGGTCTGGAGCAAATGCT-3′ | |
| L861Q | Forward: 5′-CCTCACAGCAGGGTCTTCTCTGT-3′ |
| Reverse: 5′-TCCCTGGTGTCAGGAAAATGCT-3′ | |
| EML4-ALK | Forward: 5′-CCTGAGTCACAGTGTTTGAGC-3′ |
| Reverse: 5′-TGCCAGCAAAGCAGTAGTTGG-3′ |
Primer sequence.
Prognostic assessment criteria
In total, 208 patients with GGO-associated lung adenocarcinoma were followed for 3 years, among whom nine were lost to follow-up and 199 completed follow-up. The last deadline for follow-up was September 2024.
Outcome measures
(1) The qualitative and quantitative parameters of three-dimensional CT in patients with GGO-associated lung adenocarcinoma with/without EGFR and ALK gene rearrangement were compared. (2) Qualitative and quantitative parameters of three-dimensional CT associated with EGFR and ALK gene rearrangement in patients with GGO-associated lung adenocarcinoma were identified. (3) The association between the qualitative and quantitative parameters of three-dimensional CT and the prognosis of patients with GGO-associated lung adenocarcinoma was investigated. (4) The predictive value of qualitative and quantitative parameters of three-dimensional CT in the prognosis of GGO-associated lung adenocarcinoma was evaluated.
Statistical analysis
The software SPSS 25.0 was used to analyze data. Measurement data with normal distribution and homogeneous variance are presented as ± s and were compared between the two groups using an independent sample t-test. Measurement data without normal distribution are presented as median [M (Q1, Q2)] and were compared using the Mann-Whitney U test. Count data are presented as n (%) and were compared using the χ2 test. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were employed to evaluate the prognostic value. A multicollinearity test revealed that the tolerance of meaningful quantitative and qualitative parameters in three-dimensional CT imaging of patients with EGFR and ALK mutations was >0.2, suggesting a low chance of multicollinearity. Multivariate logistic regression analysis was conducted with EGFR gene rearrangements as the dependent variable (wild-type = 1, mutant = 2) and BS (yes = 1, no = 0), PIS (yes = 1, no = 0), VBS (yes = 1, no = 0), MND (actual value), NV (actual value), ACTV (actual value), and SCP (actual value) as independent variables. P < 0.05 was considered statistically significant.
Results
Qualitative and quantitative parameters of three-dimensional CT in patients with GGO-associated lung adenocarcinoma and EGFR and ALK gene rearrangement
Among 208 patients with GGO-associated lung adenocarcinoma, 121 (58.17%) had wild-type EGFR, while 87 (41.83%) had mutated EGFR. There were two cases with exon 18 mutations, 41 cases with exon 19 deletions, 10 cases with exon 20 insertions, and 34 cases with exon 21 L858R point mutations. There were 16 positive cases (7.69%) and 192 negative cases (92.31%) for the ALK gene rearrangement. There were significant differences between patients with wild-type EGFR and patients with mutant EGFR in terms of the BS, PIS, VBS, MND, NV, ACTV, and SCP (P < 0.05). There were significant differences between ALK gene rearrangement-positive and ALK gene rearrangement-negative patients in terms of the BS, PIS, VBS, ACTV, and SCP (P < 0.05) (Table 2). Four representative images were selected (Figure 2).
TABLE 2
| Parameters | EGFR gene mutated | t/χ2/P | ALK gene rearrangement | t/χ2/P | ||
|---|---|---|---|---|---|---|
| Wild-Type (121 cases) | Mutant-Type (87 cases) | Negative (192 cases) | Positive (16 cases) | |||
| Qualitative parameters | ||||||
| Location of the lesion | 0.906/0.341 | 1.190/0.275 | ||||
| Left | 72 (59.50) | 46 (52.87) | 111 (57.81) | 7 (43.75) | ||
| Right | 49 (40.50) | 41 (47.83) | 81 (42.19) | 9 (56.25) | ||
| Type of nodule | 1.032/0.310 | 0.060/0.807 | ||||
| Simplex | 53 (43.80) | 32 (36.78) | 78 (40.63) | 7 (43.75) | ||
| Hybrid | 68 (56.20) | 55 (63.22) | 114 (59.38) | 9 (56.25) | ||
| Tumor shape | 0.878/0.349 | 2.258/0.133 | ||||
| Round/quasi-round | 63 (52.07) | 51 (58.62) | 55 (28.65) | 8 (50.00) | ||
| Irregular | 58 (47.93) | 36 (41.38) | 137 (71.35) | 8 (50.00) | ||
| Lobulation sign | 92 (76.03) | 61 (70.11) | 0.911/0.340 | 141 (73.44) | 12 (75.00) | 0.025/0.874 |
| Burr sign | 64 (52.89) | 57 (65.52) | 3.315/0.069 | 110 (57.29) | 11 (68.75) | 0.797/0.372 |
| Vacuolar sign | 26 (21.49) | 18 (20.69) | 0.019/0.889 | 39 (20.31) | 5 (31.25) | 0.505/0.477 |
| Empty | 15 (12.440) | 12 (13.79) | 0.087/0.768 | 25 (13.02) | 2 (12.50) | 0.107/0.743 |
| BS | 30 (24.79) | 39 (44.83) | 9.163/0.002 | 57 (29.69) | 12 (75.00) | 13.679/<0.001 |
| PIS | 27 (22.31) | 39 (44.83) | 11.842/0.001 | 56 (29.17) | 10 (62.50) | 7.575/0.006 |
| VBS | 29 (23.97) | 46 (52.87) | 18.342/<0.001 | 65 (33.85) | 11 (68.75) | 7.756/0.005 |
| Quantitative indicators | ||||||
| MND (mm) | 14.87 ± 4.89 | 18.83 ± 4.78 | 5.815/<0.001 | 15.24 (9.75,23.10) | 16.72 (6.62,28.78) | 2.826/0.078 |
| NV(mm3) | 485.78 ± 95.68 | 974.13 ± 268.71 | 18.445/<0.001 | 688.86 ± 173.49 | 704.20 ± 218.57 | 0.333/0.740 |
| ACTV(HU) | −608.74 ± 236.78 | −518.97 ± 165.18 | 3.043/0.003 | −657.85 ± 158.96 | −468.73 ± 125.68 | 4.636/<0.001 |
| SCP(%) | 12.68 ± 5.15 | 19.68 ± 5.67 | 9.268/<0.001 | 15.45 (6.95,32.42) | 18.78 (5.13,35.69) | 19.87 < 0.001 |
Comparison of qualitative and quantitative parameters of three-dimensional CT in patients with different EGFR and ALK gene rearrangements [n(%)/( ± s)].
FIGURE 2
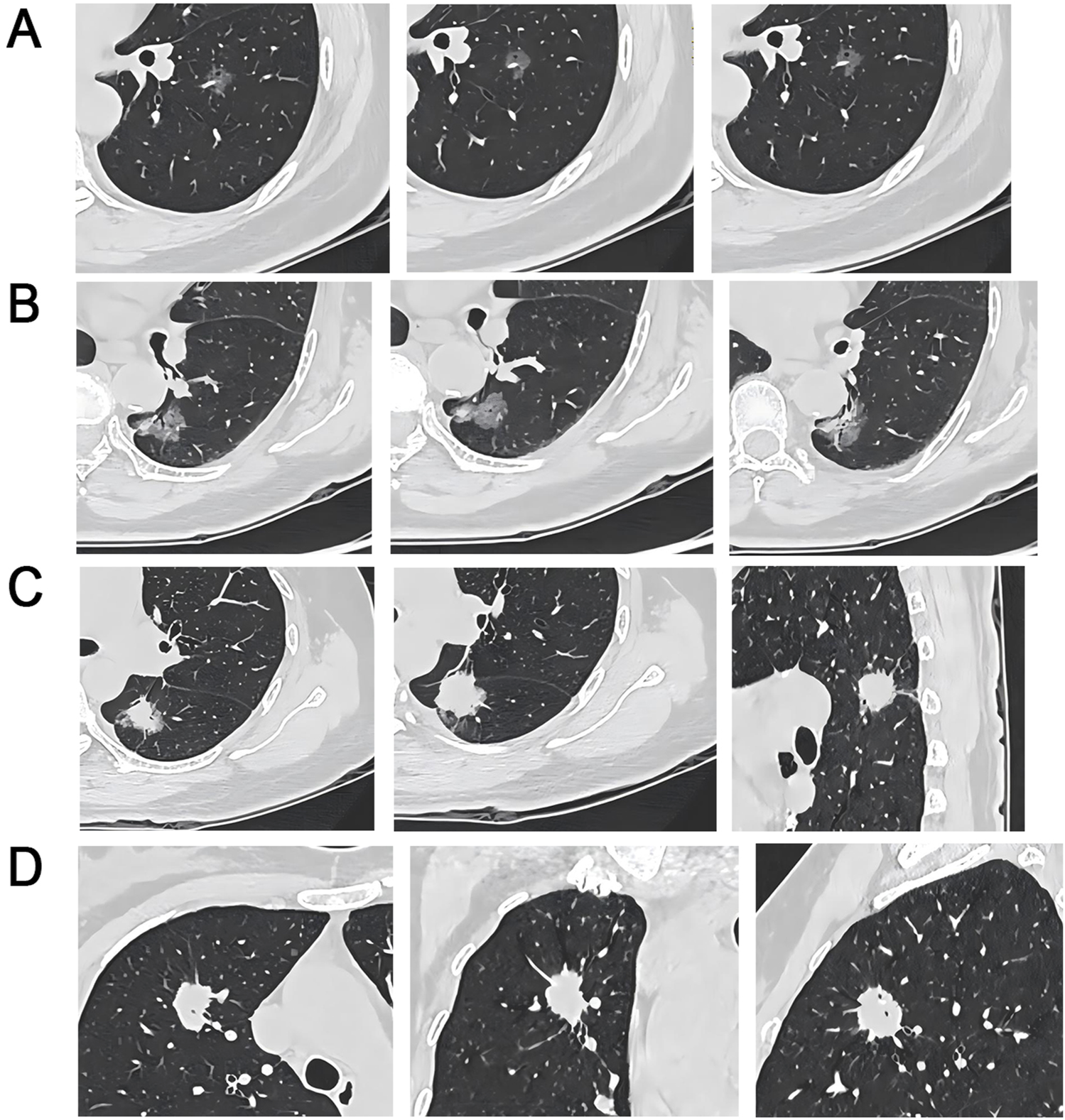
CT Imaging Characteristics of EGFR Wild-Type and ALK-Positive/Negative Lung Nodules (A) EGFR wild-type patient, 61-year-old female, pure GGO in the left lower lobe with vacuole, lobulation, spiculation, and PIS; (B) Circular EGFR wild-type, 75-year-old female, mixed GGO in the left lower lobe with pleural indentation, bronchial, vacuole, lobulation, and PIS; (C) ALK-negative, 56-year-old female, sub-solid nodule in the left lower lobe with bronchial, pleural indentation, spiculation, and deep lobulation signs; (D) ALK-positive, 51-year-old female, solid nodule in the right upper lobe with vacuole and spiculation signs.
The association between the qualitative and quantitative parameters of three-dimensional CT and EGFR and ALK gene rearrangement in GGO-associated lung adenocarcinoma
The multi-comparison multicollinearity test showed that the tolerance of meaningful quantitative and qualitative parameters in the three-dimensional CT of patients with EGFR and ALK mutations was >0.2, and the possibility of multicollinearity was small. The results indicated that BS, PIS, VBS, MND, NV, ACTV, and SCP were associated with EGFR mutation in GGO-associated lung adenocarcinoma (Table 3).
TABLE 3
| Influencing factors | β | SE | Wald x 2 | OR | 95%CI | P | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| BS | 1.394 | 0.265 | 27.675 | 4.031 | 1.856 | 8.756 | 0.010 |
| PIS | 2.316 | 0.359 | 41.614 | 10.134 | 6.789 | 15.126 | <0.001 |
| VBS | 1.598 | 0.424 | 14.233 | 4.942 | 2.246 | 10.873 | 0.006 |
| MND | 0.257 | 0.125 | 4.214 | 1.293 | 1.125 | 1.485 | <0.001 |
| NV | 0.414 | 0.149 | 7.712 | 1.513 | 1.132 | 2.021 | <0.001 |
| ACTV | −1.313 | 0.258 | 25.900 | 0.269 | 0.158 | 0.458 | <0.001 |
| SCP | 0.340 | 0.128 | 7.038 | 1.404 | 1.257 | 1.569 | 0.001 |
Multivariate logistic regression results of EGFR gene rearrangements.
Multivariate logistic regression analysis was conducted using ALK gene rearrangement status as the dependent variable (negative = 1, positive = 2) and BS, PIS, VBS, ACTV, and SCP as independent variables. The results revealed that these factors were correlated with ALK gene rearrangement in GGO-related lung adenocarcinoma (Table 4).
TABLE 4
| Influencing factors | β | SE | Wald x 2 | OR | 95%CI | P | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| BS | 1.548 | 0.357 | 18.809 | 4.703 | 2.268 | 9.754 | <0.001 |
| PIS | 1.921 | 0.478 | 16.144 | 6.825 | 3.453 | 13.489 | <0.001 |
| VBS | 1.368 | 0.302 | 20.513 | 3.927 | 1.418 | 10.874 | <0.001 |
| ACTV | −0.736 | 0.159 | 21.400 | 0.479 | 0.268 | 0.857 | <0.001 |
| SCP | 0.316 | 0.147 | 4.611 | 1.371 | 1.157 | 1.625 | 0.007 |
Multivariate logistic regression results of ALK gene rearrangement.
Prognostic prediction in GGO-associated lung adenocarcinoma based on three-dimensional CT qualitative and quantitative parameters
In total, 208 patients were followed for 3 years, among whom nine were lost to follow-up. Among 199 patients with complete follow-up, 53 suffered from recurrence, metastasis, or death and were classified as having a poor prognosis, while the remaining 146 patients were classified as having a good prognosis. There were significant differences in BS, PIS, VBS, MND, NV, ACTV, and SCP between patients with good prognosis and those with poor prognosis (P < 0.05) (Table 5).
TABLE 5
| Parameters | Favorable prognosis (146 cases) | Unfavorable prognosis (53 cases) | t/χ2/P |
|---|---|---|---|
| Qualitative parameters | |||
| Location of the lesion | 1.598/0.206 | ||
| Left | 79 (54.11) | 34 (64.15) | |
| Right | 67 (45.89) | 19 (35.85) | |
| Type of nodule | 0.886/0.347 | ||
| Simplex | 58 (39.73) | 25 (47.17) | |
| Hybrid | 88 (60.27) | 28 (52.83) | |
| Tumor shape | 1.051/0.305 | ||
| Round/quasi-round | 79 (54.11) | 33 (62.26) | |
| Irregular | 67 (45.89) | 20 (37.74) | |
| Lobulation sign | 108 (73.97) | 42 (79.25) | 0.582/0.445 |
| Burr sign | 83 (56.82) | 32 (60.38) | 0.198/0.656 |
| Vacuolar sign | 25 (17.12) | 13 (24.53) | 1.380/0.240 |
| Empty | 17 (11.64) | 8 (15.09) | 0.421/0.516 |
| BS | 38 (26.03) | 26 (49.06) | 9.452/0.002 |
| PIS | 33 (22.60) | 25 (47.17) | 11.364/0.001 |
| VBS | 39 (26.71) | 28 (52.83) | 11.877/0.001 |
| Quantitative indicators | |||
| MND (mm) | 13.285 ± 3.97 | 19.45 ± 3.69 | 9.862/<0.001 |
| NV(mm3) | 512.68 ± 102.37 | 933.18 ± 245.85 | 17.044/<0.001 |
| ACTV(HU) | −626.87 ± 189.74 | −478.21 ± 157.83 | 5.097/<0.001 |
| SCP(%) | 13.15 ± 4.02 | 17.67 ± 5.18 | 6.470/<0.001 |
Comparison of qualitative and quantitative parameters of three-dimensional CT in patients with differential prognosis [n(%)/( ± s)].
Predictive value of qualitative and quantitative parameters of three-dimensional CT for the prognosis of GGO-associated lung adenocarcinoma
The AUC for BS, PIS, VBS, MND, NV, ACTV, and SCP were 0.625, 0.651, 0.602, 0.767, 0.755, 0.751, and 0.789, respectively. The AUC for the combination of these variables was 0.914, which was significantly greater than the AUC of the individual parameters (P < 0.05) (Figure 3; Table 6).
FIGURE 3
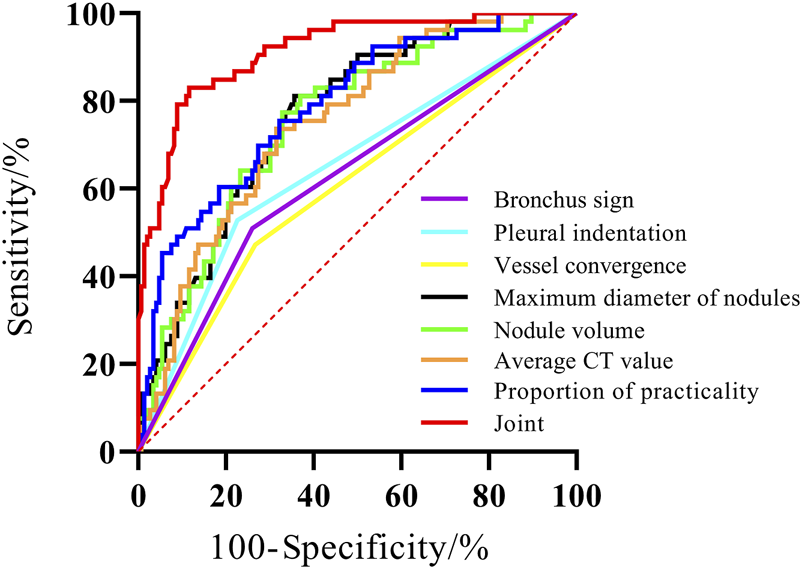
The ROC curve of three-dimensional CT qualitative and quantitative parameters on the prognosis of GGO-associated lung adenocarcinoma.
TABLE 6
| Parameters | AUC | 95%CI | Sensitivity (%) | Specificity (%) | Z | P |
|---|---|---|---|---|---|---|
| BS | 0.625 | 0.553∼0.692 | 50.94 | 73.97 | 3.181 | 0.002 |
| PIS | 0.651 | 0.581∼0.717 | 52.83 | 77.40 | 3.903 | 0.001 |
| VBS | 0.602 | 0.531∼0.671 | 47.17 | 73.29 | 2.610 | 0.009 |
| MND | 0.767 | 0.702∼0.824 | 81.13 | 64.38 | 7.539 | <0.001 |
| NV | 0.755 | 0.689∼0.813 | 77.36 | 67.12 | 6.752 | <0.001 |
| ACTV | 0.751 | 0.685∼0.810 | 73.58 | 68.49 | 6.833 | <0.001 |
| SCP | 0.789 | 0.725∼0.843 | 75.47 | 67.81 | 8.018 | <0.001 |
| united | 0.914 | 0.866∼0.949 | 83.02 | 88.36 | <0.001 |
The predictive value of qualitative and quantitative parameters of three-dimensional CT in the prognosis of GGO-associated lung adenocarcinoma.
Discussion
Previous studies have reported a relationship between ground-glass opacity (GGO) and GGO-associated lung adenocarcinoma [9]. Compared to traditional two-dimensional computed tomography (CT), three-dimensional CT reconstructions based on artificial intelligence are more effective in diagnosing GGO. They can accurately localize the anatomical structure of lung nodules, visualize the density and marginal characteristics of pulmonary nodules, and significantly improve the detection rate of GGO, especially for small-diameter nodules [10].
Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) gene rearrangements are risk factors for the progression of lung adenocarcinoma. The EGFR and ALK genes are also the primary gene targets for targeted therapy in lung adenocarcinoma. Recently, many studies have explored the relationship between EGFR and ALK gene rearrangements and the imaging and pathological features of lung cancer, but there are many controversies [7, 11]. There is no clinical study on the relationship between three-dimensional CT imaging findings and EGFR and ALK gene rearrangements or prognosis in GGO-associated lung adenocarcinoma.
In this study, 41.83% of 208 patients with early-stage lung adenocarcinoma had EGFR gene rearrangements and 7.69% had ALK gene rearrangements. Meiying et al. [12] reported an EGFR mutation rate of 59.41% and an ALK rearrangement rate of 3.66%, which differ from our findings. These discrepancies can be attributed to several factors, including differences in sample size. Their study analyzed 547 patients with invasive lung adenocarcinoma, whereas our study included 208 patients with GGO-associated lung adenocarcinoma, encompassing both pre-invasive and invasive lesions. Moreover, differences in the maximum tumor diameter reflect variations in disease staging and inclusion criteria. Clinical characteristics of patients, such as age distribution, smoking status, and histological subtypes (e.g., acinar, papillary, and solid patterns), may also contribute to these discrepancies. Additionally, variations in genetic testing methods and sensitivity might have affected the detection rates of gene mutations. Furthermore, racial and regional factors, including genetic predisposition and environmental exposures, may also play a significant role in this discrepancy. Given the complexity of these influencing factors, further studies are needed to elucidate their effects on gene mutation and rearrangement rates, thereby enhancing our understanding of their clinical significance.
Further analysis of the qualitative and quantitative parameters of three-dimensional CT in patients with different EGFR and ALK gene rearrangements revealed that the BS, PIS, VBS, MND, NV, ACTV, and SCP were correlated with EGFR mutation in GGO-associated lung adenocarcinoma. These findings are consistent with those of Liu Y et al. [13]. Zhu Na et al. [14] found that there were no significant differences in the MND, BS, PIS, and VBS between patients with EGFR mutation and those with wild-type EGFR. These discrepancies can be attributed to differences in the incidence of wild-type and mutant EGFR, smoking proportions, and histological subtypes. This study also found that the BS, PIS, VBS, ACTV, and SCP were correlated with the presence of ALK gene rearrangement in GGO-related lung adenocarcinoma, which is inconsistent with the findings of Han XY et al. [15]. The inconsistencies might be related to differences in the solid tumor volume of patients and the proportion of mucinous lung adenocarcinoma.
The EGFR gene, which encodes a transmembrane tyrosine kinase, is a commonly mutated gene in lung adenocarcinoma and is more frequently observed in elderly patients (≥60 years old), with a mutation rate of approximately 15%–50% [16]. The ALK gene is also commonly mutated in lung adenocarcinoma, with a mutation rate of approximately 3%–7%. ALK gene rearrangement is associated with tumor cell proliferation, differentiation, migration, neoangiogenesis, and apoptosis, making it an important target for treating lung adenocarcinoma [17]. Compared to EGFR mutations, which are more commonly observed in elderly patients, ALK rearrangements are predominantly found in younger patients (<60 years old), non-smokers, or light smokers with lung adenocarcinoma [6]. In malignant tumor cells, the pleural depression sign can stimulate tumor cells to release growth and signaling factors, promoting the proliferation and differentiation of fibroblasts in surrounding tissues and accelerating the growth of malignant tumors [18]. The VBS refers to the excessive proliferation of cancer cells in the lesion, resulting in irregular angiogenesis and cluster distribution, which is related to tumor growth, differentiation, and migration. It can also promote angiogenesis [19]. BS is related to tumors that surround the bronchial tubes but do not obstruct them. They are more pronounced when the fibrous fibers in the lesion shrink [20]. Compared to morphological characteristics, the quantitative parameters of three-dimensional CT can more accurately and efficiently determine the relationship between the CT characteristics of the lesions and gene rearrangements. Changes in the MND, NV, ACTV, and SCP are associated with tumor proliferation and differentiation, invasion, and vascular density [6, 21]. The progression of lung adenocarcinoma thickens the local lung interstitium inside the lesion, and cancer cells metastasize into the alveoli, thereby increasing tumor density, promoting tumor growth and metastasis, and accelerating tumor progression [22].
Several studies have confirmed that CT imaging findings are closely associated with the prognosis of GGO lung adenocarcinoma [23]. Wan GY et al. [24] found that the ACTV, SCP, and BS are predictors of disease-free survival in patients with lung adenocarcinoma. This study found significant differences in BS, PIS, VBS, MND, NV, ACTV, and SCP among patients with different prognoses.
In the study conducted by Wan GY et al., the maximum diameter of the lesion was ≤50 mm. The presence of satellite lesions, tiny calcifications, insufficient blood supply, increased formation of new blood vessels, rapid malignant proliferation, and a high degree of tumor malignancy were observed. ROC curves revealed that three-dimensional CT qualitative and quantitative parameters alone had poor predictive ability for the prognosis of GGO-associated lung adenocarcinoma, with low sensitivity or specificity. The AUC of the joint prediction model was 0.914, with the best sensitivity and specificity being 83.02% and 88.36%, respectively. This finding indicates that the combination of BS, PIS, VBS, MND, NV, ACTV, and SCP can predict the prognosis of GGO-associated lung adenocarcinoma, thereby helping to identify patients with a poor prognosis and improve their outcomes.
This study has several limitations. First, it focused solely on EGFR and ALK mutations without analyzing other potentially relevant driver genes, and the use of peripheral blood for detecting these genetic alterations may have increased false-negative rates. Second, the relatively short follow-up period restricted our ability to assess long-term prognosis and fully capture the evolutionary process of GGO-associated lung adenocarcinoma. Third, the single-center design and limited sample size may compromise the generalizability of the findings. Additionally, the study exclusively included patients with GGO-associated lung adenocarcinoma, excluding those without GGO, which limits the broader applicability of the results. Fourth, we focused solely on EGFR and ALK gene alterations and did not include other driver mutations. Although prior studies suggest a low K-ras mutation rate in GGO-dominant lung adenocarcinoma and mutual exclusivity between EGFR/ALK and K-ras alterations, future research should expand genetic profiling to assess the impact of multi-gene interactions on CT imaging features.
In summary, the quantitative and qualitative parameters of three-dimensional CT are related to EGFR and ALK gene rearrangements and prognosis in patients with GGO-associated lung adenocarcinoma. The combination of BS, PIS, VBS, MND, NV, ACTV, and SCP can help predict the prognosis of patients and formulate subsequent treatment plans.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Chongqing Universal Hongling Medical Imaging Diagnostic Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL: Conceptualization, Methodology, Writing-original draft, Writing-review and editing, Supervision, Project administration; YR: Formal analysis, Writing-review and editing; ZF: Investigation, Writing-review and editing; QS: Resources, Writing-review and editing; LL: Validation, Writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Zhang X Yang L Liu S Cao LL Wang N Li HC et al Interpretation on the report of global cancer statistics 2022. Chin J Oncol (2024) 46(7):710–21. 10.3760/cma.j.cn112152-20240416-00152
2.
Hattori A Matsunaga T Fukui M Takamochi K Suzuki K . Prognostic influence of a ground-glass opacity component in hypermetabolic lung adenocarcinoma. Eur J Cardiothorac Surg (2022) 61(2):249–56. 10.1093/ejcts/ezab436
3.
Chen ZH Chen ZY Kang J Chu XP Fu R Zhang JT et al Investigation on the incidence and risk factors of lung cancer among Chinese hospital employees. Thorac Cancer (2022) 13(15):2210–22. 10.1111/1759-7714.14549
4.
Mizuno T Terada Y Katsumata S Konno H Nagata T Isaka M et al Diagnostic sensitivity of solid volume measurement for pathological invasion in non-solid lung adenocarcinoma. J Thorac Dis (2023) 15(6):2916–25. 10.21037/jtd-22-1603
5.
Park SJ Ju S Goh SH Yoon BH Park JL Kim JH et al Proteogenomic characterization reveals estrogen signaling as a target for never-smoker lung adenocarcinoma patients without EGFR or ALK alterations. Cancer Res (2024) 84(9):1491–503. 10.1158/0008-5472.CAN-23-1551
6.
Cai Y Chen T Zhang SJ Tan M Wang J . Correlation exploration among CT imaging,pathology and genotype of pulmonary ground-glass opacity. J Cell Mol Med (2023) 27(14):2021–31. 10.1111/jcmm.17797
7.
Igde MH Ozturk A Oruc O Oztas S Kavas M . The relationship of PET/CT SUVmax with EGFR mutation status and ALK rearrangement in lung adenocarcinoma. Hell J Nucl Med (2022) 25(2):188–95. 10.1967/s002449912486
8.
Zhi XY Shi YK Yu JM . Chinese standard for the diagnosis and treatment of primary lung cancer. Chin J Oncol (2015) 37(1):67–78. 10.3760/cma.j.issn.0253-3766.2015.01.014
9.
Lee JH Choi Y Hong H Kim YT Goo JM Kim H . Prognostic value of CT-defined ground-glass opacity in early-stage lung adenocarcinomas: a single-center study and meta-analysis. Eur Radiol (2024) 34(3):1905–20. 10.1007/s00330-023-10160-x
10.
Jing HN Yao CY Chen YW . Application of three-dimensional CT quantitative analysis in the early diagnosis and invasive evaluation of pulmonary mixed ground-glass nodules. Chin J CT MRI (2023) 21(9):76–8. 10.3969/j.issn.1672-5131.2023.09.026
11.
Masson-Grehaigne C Lafon M Palussière J Leroy L Bonhomme B Jambon E et al Single- and multi-site radiomics May improve overall survival prediction for patients with metastatic lung adenocarcinoma. Diagn Interv Imaging (2024) 105(11):439–52. 10.1016/j.diii.2024.07.005
12.
Cheng MY Wang YL Wang X . Influence of EGFR gene mutation subtypes and ALK gene mutation on clinicopathological features of invasive lung adenocarcinoma. J Mod Oncol (2023) 31(20):3791–5. 10.3969/j.issn.1672-4992.2023.20.013
13.
Liu Y Kim J Qu FY Liu S Wang H Balagurunathan Y et al CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology (2016) 280(1):271–80. 10.1148/radiol.2016151455
14.
Zhu N Zhang CY Ma N . Correlation between CT signs of pulmonary adenocarcinoma appearing as ground-glass nodule and epidermal growth factor receptor gene expression. Chin J Med Imaging (2021) 29(11):1095–9. 10.3969/j.issn.1005-5185.2021.11.009
15.
Han XY Fan J Li YM Cao Y Gu J Jia X et al Value of CT features for predicting EGFR mutations and ALK positivity in patients with lung adenocarcinoma. Sci Rep (2021) 11(1):5679. 10.1038/s41598-021-83646-7
16.
Wu SG Chang YL Yu CJ Yang PC Shih JY . Lung adenocarcinoma patients of young age have lower EGFR mutation rate and poorer efficacy of EGFR tyrosine kinase inhibitors. ERJ Open Res (2017) 3(3):00092-2016. 10.1183/23120541.00092-2016
17.
Shaw AT Yeap BY Mino-Kenudson M Digumarthy SR Costa DB Heist RS et al Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol (2009) 27(26):4247–53. 10.1200/JCO.2009.22.6993
18.
Liang ZR Lv FJ Fu BJ Lin RY Li WJ Chu ZG . Reticulation sign on thin-section CT: utility for predicting invasiveness of pure ground-glass nodules. AJR Am J Roentgenol (2023) 221(1):69–78. 10.2214/AJR.22.28892
19.
Liu CM He YZ Luo JM . Application of chest CT imaging feature model in distinguishing squamous cell carcinoma and adenocarcinoma of the lung. Cancer Manag Res (2024) 16(1):547–57. 10.2147/CMAR.S462951
20.
Li Q Fan X Huo JW Luo TY Huang XT Gong JW . Differential diagnosis of localized pneumonic-type lung adenocarcinoma and pulmonary inflammatory lesion. Insights Imaging (2022) 13(1):49. 10.1186/s13244-022-01200-z
21.
Qiu YH Hao YJ Pei YB . Diagnostic value of CT three-dimensional parameters on invasiveness of pulmonary groundglass nodules smaller than 2 cm. Acad J Chin PLA Med Sch (2023) 44(6):607–12. 10.3969/j.issn.2095-5227.2023.06.006
22.
Messa F Siciliani A Piccioni G Leonardi B Ciccone AM D'Andrilli A et al Prognostic factors of non-predominant-lepidic lung adenocarcinoma presenting as ground glass opacity: results of a multicenter study. J Pers Med (2024) 14(2):153. 10.3390/jpm14020153
23.
Park S Lee SM Choe J Choi S Do KH Seo JB . Recurrence patterns and patient outcomes in resected lung adenocarcinoma differ according to ground-glass opacity at CT. Radiology (2023) 307(3):e222422. 10.1148/radiol.222422
24.
Wan GY Kong JJ Zhang L . The degree of tissue differentiation and prognostic significance of lung adenocarcinoma based on CT imaging features. Oncoradiology (2024) 33(04):388–94. 10.19732/j.cnki.2096-6210.2024.04.008
Summary
Keywords
three-dimensional CT, ground-glass opacities, lung adenocarcinoma, EGFR, ALK
Citation
Liu X, Ren Y, Fei Z, Shi Q and Lu L (2025) Relationship between qualitative and quantitative parameters of three-dimensional computed tomography, EGFR gene mutation, and ALK gene rearrangement in GGO-associated lung adenocarcinoma and their prognostic value. Pathol. Oncol. Res. 31:1612081. doi: 10.3389/pore.2025.1612081
Received
15 January 2025
Accepted
11 August 2025
Published
11 September 2025
Volume
31 - 2025
Edited by
Anna Sebestyén, Semmelweis University, Hungary
Updates
Copyright
© 2025 Liu, Ren, Fei, Shi and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyan Liu, wcs876490@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.