Abstract
Purpose:
To clarify the prognostic value of lymph node regression (LNR) status including the lymph node regression grade (LNRG) and N downstaging in patients with esophageal cancer receiving neoadjuvant therapy based on available evidence.
Methods:
Several databases were searched up to 25 March 2024. The main outcomes included overall survival (OS), disease-free survival (DFS) and cancer-specific survival (CSS). Hazard ratios (HRs) and 95% confidence intervals (CIs) were combined. Subgroup analyses based on the neoadjuvant therapy and pathological type were also conducted.
Results:
In total, 14 retrospective studies with 3,212 participants were included. Nine and five studies explored the relationship between LNRG and N downstaging and survival, respectively. Pooled results indicated that complete LNR predicted significantly improved OS (HR = 0.47, 95% CI: 0.41–0.55, P < 0.001) and DFS (HR = 0.42, 95% CI: 0.32–0.55, P < 0.001) and subgroup analysis based on neoadjuvant therapy and pathological type manifested similar results. Besides, N downstaging was also significantly related to improved OS (HR = 0.40, 95% CI: 0.21–0.77, P = 0.006) and CSS (HR = 0.27, 95% CI: 0.12–0.60, P < 0.001).
Conclusion:
LNR could serve as a novel and reliable prognostic factor in patients with esophageal cancer receiving neoadjuvant therapy and complete LNR and N downstaging predict better survival.
Introduction
Esophageal cancer ranks as the fourth leading cause of cancer-related deaths, comprising predominantly squamous cell carcinoma and adenocarcinoma, with an overall poor prognosis [1, 2]. In recent years, the clinical role of neoadjuvant therapy in the treatment of esophageal cancer has become increasingly prominent and neoadjuvant therapy combined with surgery significantly improves the prognosis for the majority of patients with locally advanced esophageal cancer [3]. Recent studies have demonstrated the superiority of neoadjuvant chemoradiotherapy over neoadjuvant chemotherapy in terms of long-term survival outcomes in locally advanced esophageal cancer [4]. Even with similar adverse effects, neoadjuvant chemoradiotherapy provides superior short-term benefits compared to neoadjuvant chemotherapy [4]. Moreover, neoadjuvant chemoradiotherapy is associated with impressive rates of pathological complete response, and survival [5, 6]. In addition, the role of neoadjuvant immunotherapy is gradually being elucidated in the clinic [7]. Thus, neoadjuvant therapy holds an indispensable position in the comprehensive antitumor therapy of esophageal cancer.
Furthermore, with the incorporation of adjuvant therapy, the TNM stage appears to be less reliable in predicting the prognosis of neoadjuvant therapy compared to surgery alone. Therefore, to aid in prognosis evaluation, imaging and pathological approaches to assess tumor regression are considered vital evaluation tools. However, pathological criteria undoubtedly offer greater objectivity. The widely adopted tumor regression grade (TRG) system categorizes residual tumors after treatment-induced regression based on parameters such as quantity, proportion, tumor cell condition, and extent and distribution of fibrosis [8]. Nevertheless, there exist multiple variations of TRG [9, 10]. While the majority of evaluation systems are generally regarded as good indicators for predicting short-term therapeutic effects and estimating the risk of recurrence, each grade only reflects the therapeutic effect on the primary tumors [9, 10]. However, it has been reported that the response of primary lesions and lymph nodes to neoadjuvant therapy differs [9]. Furthermore, some studies have indicated that lymph node metastasis status is more strongly associated with prognosis than primary lesion invasion, whether after surgery or neoadjuvant therapy [11]. Consequently, lymph nodes must be individually evaluated and the prognostic role of lymph node regression (LNR) in patients with esophageal cancer should be further determined. For LNR grade (LNRG) assessment, dissected lymph nodes are usually stained with hematoxylin and eosin and analyzed microscopically for metastatic disease in clinics. Currently, there is no standardized protocol for evaluating LNR. Lymph node downstaging is usually defined as any regional lymph node that is positive on clinical evaluation (cN+) and subsequently has no evidence of pathologic regional lymph node disease. To date, the prognostic role of LNR in esophageal cancer patients undergoing neoadjuvant therapy remains uncertain.
Therefore, this study aimed to identify the predictive role of LNR status in locally advanced patients with esophageal cancer who received neoadjuvant therapy based on current evidence.
Materials and methods
This study was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses 2020 [12].
Literature search
The Medline, EMBASE, and Web of Science databases were searched from their inception up to 25 March 2024. The following terms were used: esophageal, esophagus, tumor, cancer, neoplasm, carcinoma, survival, prognostic, prognosis, neoadjuvant, lymph node regression, and N downstaging. A detailed search strategy in Medline is provided in Supplementary file 1. References to the included studies were also reviewed.
Inclusion criteria
Studies were included if they met the following criteria: 1) patients were pathologically diagnosed with primary esophageal cancer; 2) patients received neoadjuvant therapy including chemotherapy, radiotherapy, immunotherapy, or combined therapy; 3) lymph node status was evaluated before and after neoadjuvant therapy; 4) patients in the complete/subtotal response group and partial/no response group and patients in the N downstaging group (ypN0) and ypN+ group were compared separately; 5) overall survival (OS), disease-free survival (DFS) or (and) was compared between groups; 6) hazard ratios (HRs) with 95% confidence intervals (CIs) were reported, or the Kaplan-Meier survival curves were provided; 7) full texts were available in English.
Exclusion criteria
Studies were excluded if they met the following criteria: 1) insufficient, duplicated, or overlapping data; 2) animal studies, editorials, letters, meeting abstracts or case reports.
Data extraction
The following information was collected: the first author, publication year, country, sample size, c-tumor-node-metastasis (cTNM) stage, type of neoadjuvant therapy, pathological type, type of LNR, definition of LNRG or N downstaging, source of HR, follow-up time, outcome, and HR with 95% CI.
Quality evaluation
The Newcastle-Ottawa scale (NOS) was used to evaluate the quality of included retrospective studies and studies with an NOS score ≥6 were regarded as high-quality studies [13].
The literature search, selection, data extraction, and quality evaluation were all performed by two authors independently and all disagreements were resolved by team discussion.
Statistical analysis
All statistical analyses were conducted using STATA 15.0 software. HR with 95% CI was combined to identify the association between LNR and survival in patients with esophageal cancer undergoing neoadjuvant therapy. Heterogeneity among included studies was evaluated by I2 statistics and Q tests. When significant heterogeneity was observed, presenting as I2 > 50% or P < 0.1, the random-effects model was applied; otherwise, the fixed-effects model was used [14]. Subgroup analysis focusing on neoadjuvant therapy and pathological type was also conducted. Sensitivity analysis was conducted to evaluate the stability of the pooled results. Begg’s funnel plot and Egger’s test were conducted to detect publication bias [15, 16]. Significant publication bias was defined as P < 0.05.
Results
Literature search and selection
As shown in Figure 1, 690 records were identified from three databases and 189 duplicate records were removed. After reviewing the titles and abstracts, 491 records were excluded. Finally, 14 studies were included after reviewing the full text of the remaining publications [17–30]. Notably, eight studies were included in a previous similar meta-analysis by Hagens et al. [31]. Therefore, six new studies were included in this updated meta-analysis.
FIGURE 1
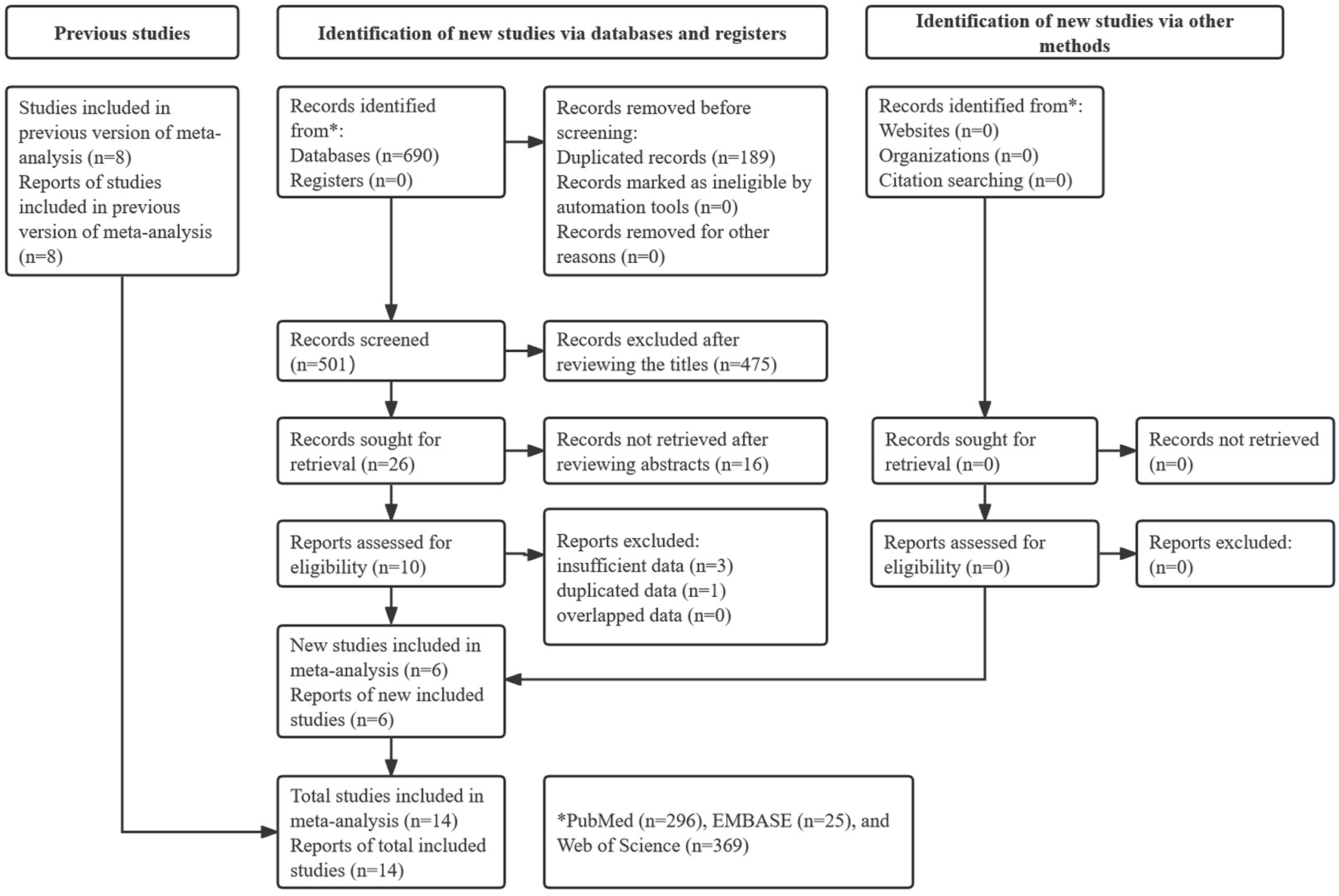
Prisma flow diagram of this meta-analysis.
Baseline characteristics of included studies
Among the 14 included retrospective studies, 3,212 patients were enrolled. Nine studies explored the relationship between LNRG and prognosis [18, 20, 21, 25–30], while the other five studies identified the predictive role of N downstaging for survival [17, 19, 22–24]. The sample sizes ranged from 40 to 981 subjects. The majority of patients received chemoradiotherapy as neoadjuvant therapy. Notably, neoadjuvant therapy was not incorporated in all included studies. All included studies directly reported HRs with 95% CIs and were high-quality studies with the NOS score ≥6. Further detailed information is presented in Table 1.
TABLE 1
| Author | Year | Country | Sample size | cTNM stage | Neoadjuvant therapy | Pathological type | LNR type | Definition of LNRG/N downstaging | Source of HR | Follow-up period | Endpoint | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bollschweiler [18] | 2011 | Germany | 40 | cT3/4N+ | CRT | Mixed | LNRG | Low-risk: no LNM and less than three LNs with central fibrosis; medium-risk: no LNM and three or more LNs with central fibrosis or LNM with an LN ratio less than 0.05; high-risk: LNM | R | 5.5 (4.2–7.6) years (median) | OS | 6 |
| Nieman [20] | 2015 | USA | 90 | cIB-cIIIA | CRT | AC | LNRG | Complete response: without evidence of metastatic disease; partial response: with evidence of previous cancer involvement but no currently viable cancer cells; no response: involved with malignancy | R | 27 (13.4–40.1) months (median) | OS | 7 |
| Philippron [21] | 2016 | Germany | 222 | cT3N + M0 | CRT | Mixed | LNRG | High-LN response: without LNM showing central fibrosis in fewer than three LNs; medium-LN response: central fibrosis in three or more LNs of ypN0 or LNM with an LN ratio of less than 0.05; Low-LN response: other | R | 4.5 (1.0–11.2) years (median) | OS | 7 |
| Davies [25] | 2018 | Sweden | 183 | cT2-4N+ | CT | AC | LNRG | Score 1: complete response; Score 2: <10% residual tumor; Score 3: 10%–50% residual tumor; Score 4: >50% viable tumor; Score 5: no response | R | NR | OS, DFS | 7 |
| Hsu [26] | 2021 | China | 115 | cT1-4N−/+ | CRT | SCC | LNRG | Score 0: N (−) with no evidence of tumor involvement or regression; score 1: N (−) with evidence of complete regression; score 2: N (+) with\50% viable tumor; score 3: N (+) with >50% viable tumor | R | NR | OS, DFS | 7 |
| Koemans [27] | 2021 | Netherlands | 117 | cIB–IIIC | CRT | Mixed | LNRG | Class A: no tumor, no signs of regression; B: tumor without regression; C: viable tumor and regression; D: complete response | R | 37 (29–44) months (median) | OS | 7 |
| Evans [28] | 2022 | Italy | 130 | cT2-4N+ | CT | AC | LNRG | Complete response: any of the following features with no residual tumor cells: the presence of foamy or hemosiderin-laden macrophages with or without dystrophic calcification, (ii) lakes of acellular mucin, and/or (iii) the presence of substantial fibrosis or tumor necrosis; partial response: with features of nodal regression and persistent tumor cells; no response: with LN metastasis but no features of regression | R | NR | OS, DFS | 7 |
| Moore [29] | 2023 | UK | 763 | cT1-4N−/+ | CT | AC | LNRG | Score 1: complete response; score 2: <10% residual tumor; score 3: 10%–50% residual tumor; score 4: >50% residual tumor; score 5: no response | R | NR | OS, DFS | 8 |
| Yehan [30] | 2024 | China | 112 | cN+ | CRT | SCC | LNRG | Score 1: 0% of total viable tumor area; score 2: <10% of total viable tumor area; score 3: 10%–50% of total viable tumor area; score 4: >50% of total viable tumor area | R | 29.6 months (median) | DFS | 7 |
| Rice [17] | 2001 | USA | 69 | cN+ | NR | Mixed | N downstaging | cN1 to ypN0 | R | 26 ± 15 months (mean) | OS | 6 |
| Donohoe [19] | 2013 | Ireland | 155 | cN+ | CRT | Mixed | N downstaging | cN+ to ypN0 | R | 48 (6–273) months (median) | OS | 6 |
| Zanoni [22] | 2016 | Italy | 55 | cN+ | CRT | Mixed | N downstaging | cN+ to ypN0 | R | 44 (11–131) months (median) | OS, CSS | 6 |
| Noble [23] | 2017 | UK | 981 | NR | CRT | AC | N downstaging | cN+ to ypN0 | R | NR | OS | 6 |
| Shapiro [24] | 2017 | Netherland | 180 | cN+ | CRT | Mixed | N downstaging | cN+ to ypN0 | R | NR | OS | 6 |
Basic characteristics of the included studies.
TNM: tumor-node-metastasis; CRT: chemoradiotherapy; CT: chemotherapy; LNR: lymph node regression; LNRG: lymph node regression grade; LNM: lymph node metastasis; LN: lymph node; AC: adenocarcinoma; SCC: squamous cell carcinoma; R: reported; OS: overall survival; DFS: disease-free survival; CSS: cancer-specific survival; NOS: Newcastle-Ottawa Scale.
Association of LNRG with survival in patients with esophageal cancer receiving neoadjuvant therapy
The survival of patients with complete (or subtotal) lymph node response and with partial (or no) lymph node response was compared in the included studies. Eight studies explored the predictive role of LNRG for OS, and the pooled results demonstrated that complete LNR predicted significantly improved OS (HR = 0.47, 95% CI: 0.41–0.55, P < 0.001; I2 = 19.4%, P = 0.276) (Figure 2). Then, subgroup analysis stratified by neoadjuvant therapy (chemoradiotherapy: HR = 0.49, 95% CI: 0.41–0.59, P < 0.001; chemotherapy: HR = 0.43, 95% CI: 0.33–0.58, P < 0.001) and pathological type (adenocarcinoma: HR = 0.46, 95% CI: 0.38–0.56, P < 0.001; squamous cell carcinoma: HR = 0.47, 95% CI: 0.27–0.82, P = 0.008) showed similar results (Table 2).
FIGURE 2
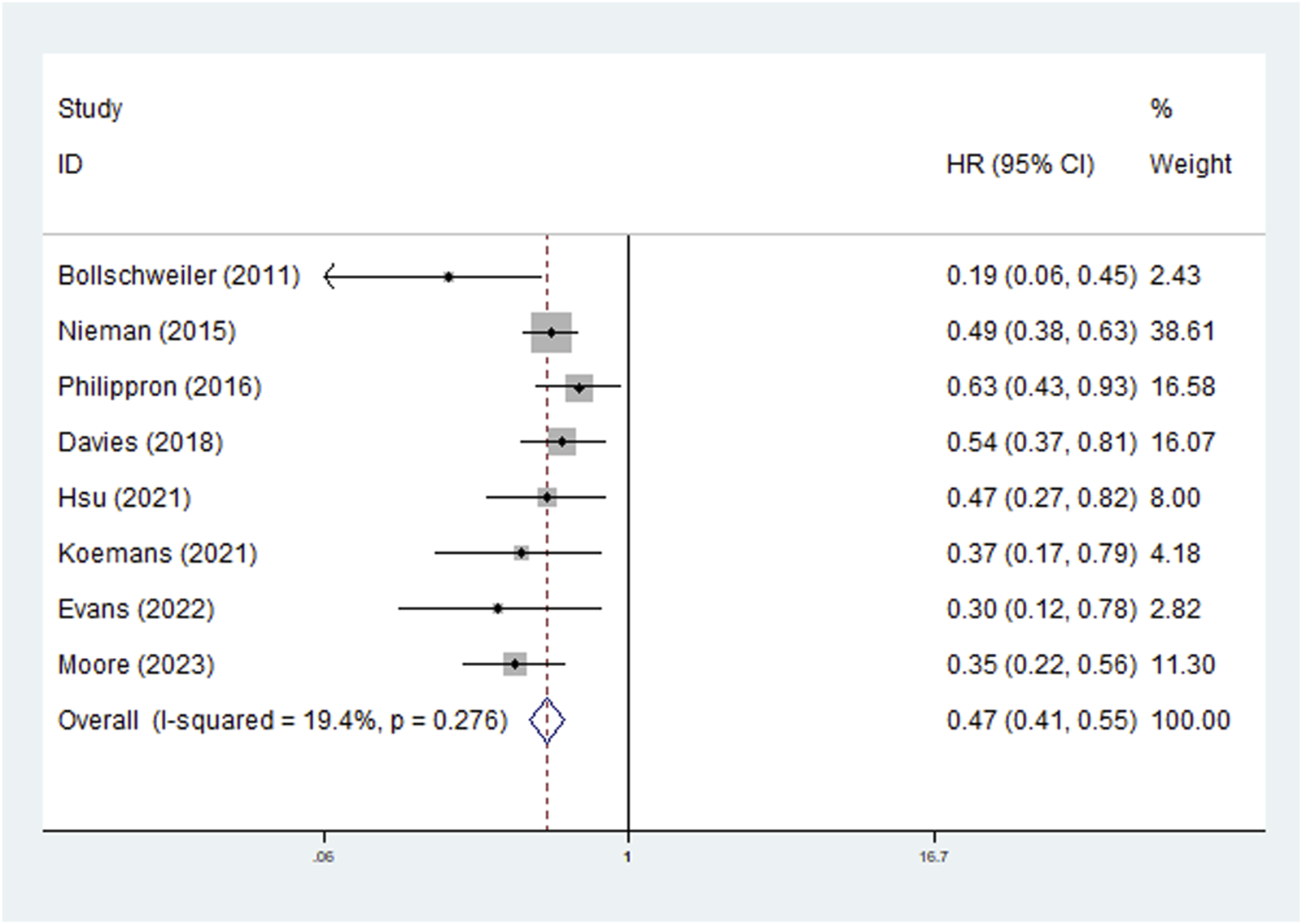
The association between lymph node regression grade and overall survival in patients with esophageal cancer undergoing neoadjuvant therapy.
TABLE 2
| No. of studies | HR | 95% CI | p-Value | I2 (%) | Pheterogeneity | |
|---|---|---|---|---|---|---|
| LNRG | ||||||
| Overall survival | 8 | 0.47 | 0.41–0.55 | <0.001 | 19.4 | 0.276 |
| Type of neoadjuvant therapy | ||||||
| Chemoradiotherapy | 5 | 0.49 | 0.41–0.59 | <0.001 | 28.1 | 0.234 |
| Chemotherapy | 3 | 0.43 | 0.33–0.58 | <0.001 | 23.3 | 0.271 |
| Pathological type | ||||||
| Adenocarcinoma | 4 | 0.46 | 0.38–0.56 | <0.001 | 0.0 | 0.394 |
| Squamous cell carcinoma | 1 | 0.47 | 0.27–0.82 | 0.008 | - | - |
| Disease-free survival | 5 | 0.42 | 0.32–0.55 | <0.001 | 30.0 | 0.221 |
| Type of neoadjuvant therapy | ||||||
| Chemoradiotherapy | 2 | 0.53 | 0.33–0.87 | 0.011 | 70.9 | 0.064 |
| Chemotherapy | 3 | 0.38 | 0.28–0.52 | <0.001 | 0.0 | 0.605 |
| Pathological type | ||||||
| Adenocarcinoma | 2 | 0.53 | 0.33–0.87 | 0.011 | 70.9 | 0.064 |
| Squamous cell carcinoma | 3 | 0.38 | 0.28–0.52 | <0.001 | 0.0 | 0.605 |
| N downstaging | ||||||
| Overall survival | 5 | 0.40 | 0.21–0.77 | 0.006 | 96.6 | <0.001 |
| Cancer-specific survival | 1 | 0.27 | 0.12–0.60 | <0.001 | - | - |
Results of the meta-analysis.
HR, hazard ratio; CI, confidence interval.
Five studies explored the relationship between LNRG and DFS and the pooled results indicated that LNRG was significantly associated with DFS (HR = 0.42, 95% CI: 0.32–0.55, P < 0.001; I2 = 30.0%, P = 0.221) (Figure 3). Similarly, subgroups based on neoadjuvant therapy (chemoradiotherapy: HR = 0.53, 95% CI: 0.33–0.87, P = 0.011; chemotherapy: HR = 0.38, 95% CI: 0.28–0.52, P < 0.001) and pathological type (adenocarcinoma: HR = 0.53, 95% CI: 0.33–0.87, P = 0.011; squamous cell carcinoma: HR = 0.38, 95% CI: 0.28–0.52, P < 0.001) identified the association of complete LNR with improved DFS (Table 2).
FIGURE 3
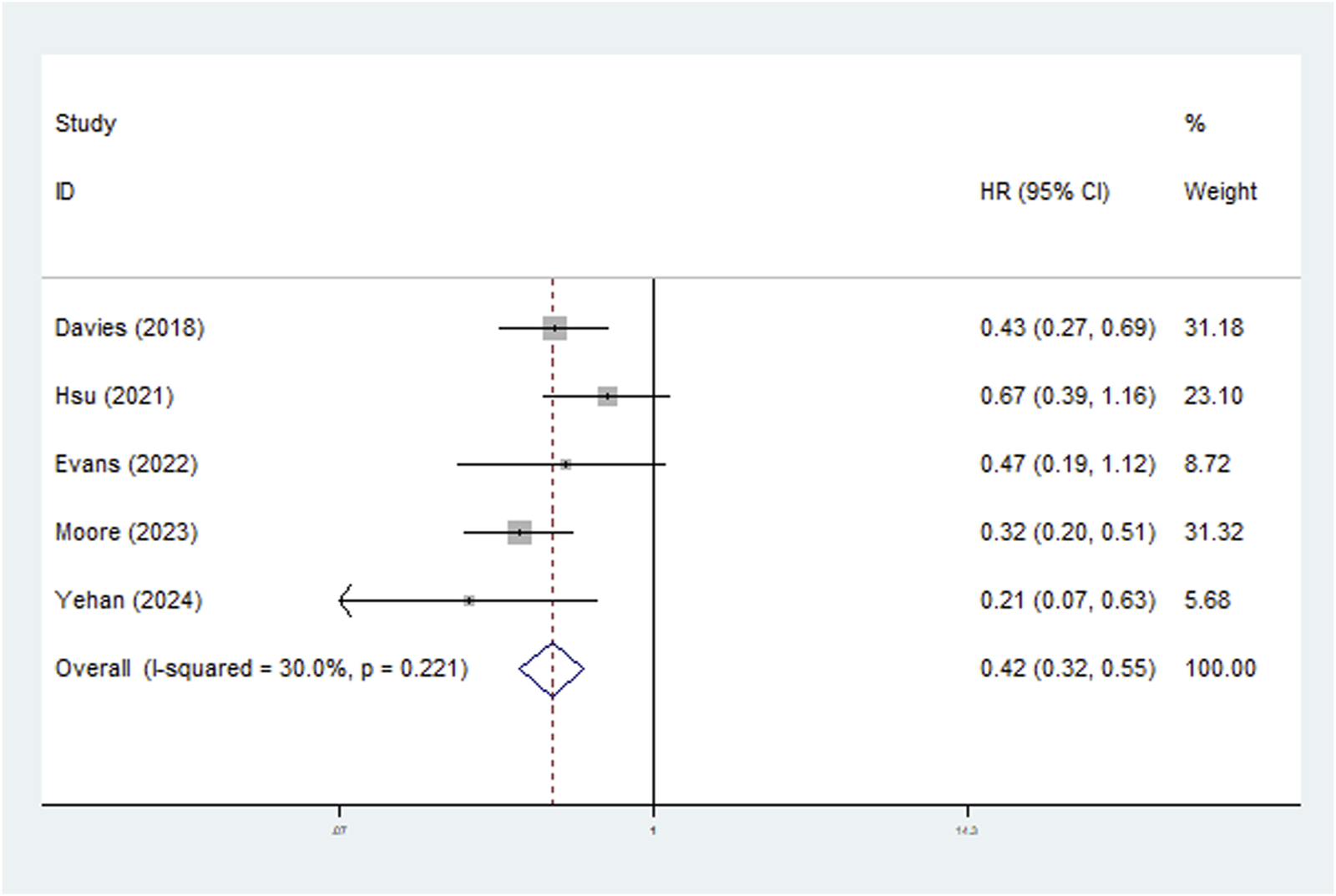
The association between lymph node regression grade and disease-free survival in patients with esophageal cancer undergoing neoadjuvant therapy.
Association of N downstaging with survival in patients with esophageal cancer receiving neoadjuvant therapy
All included studies defined the N downstaging as cN+ to ypN0. Five studies clarified the predictive role of N downstaging for OS and the pooled results demonstrated that N downstaging predicted significantly worse OS (HR = 0.40, 95% CI: 0.21–0.77, p = 0.006; I2 = 96.6%, P < 0.001) (Figure 4). However, due to the limited data available, it was not possible to conduct a more detailed subgroup analysis on N downstaging for OS.
FIGURE 4
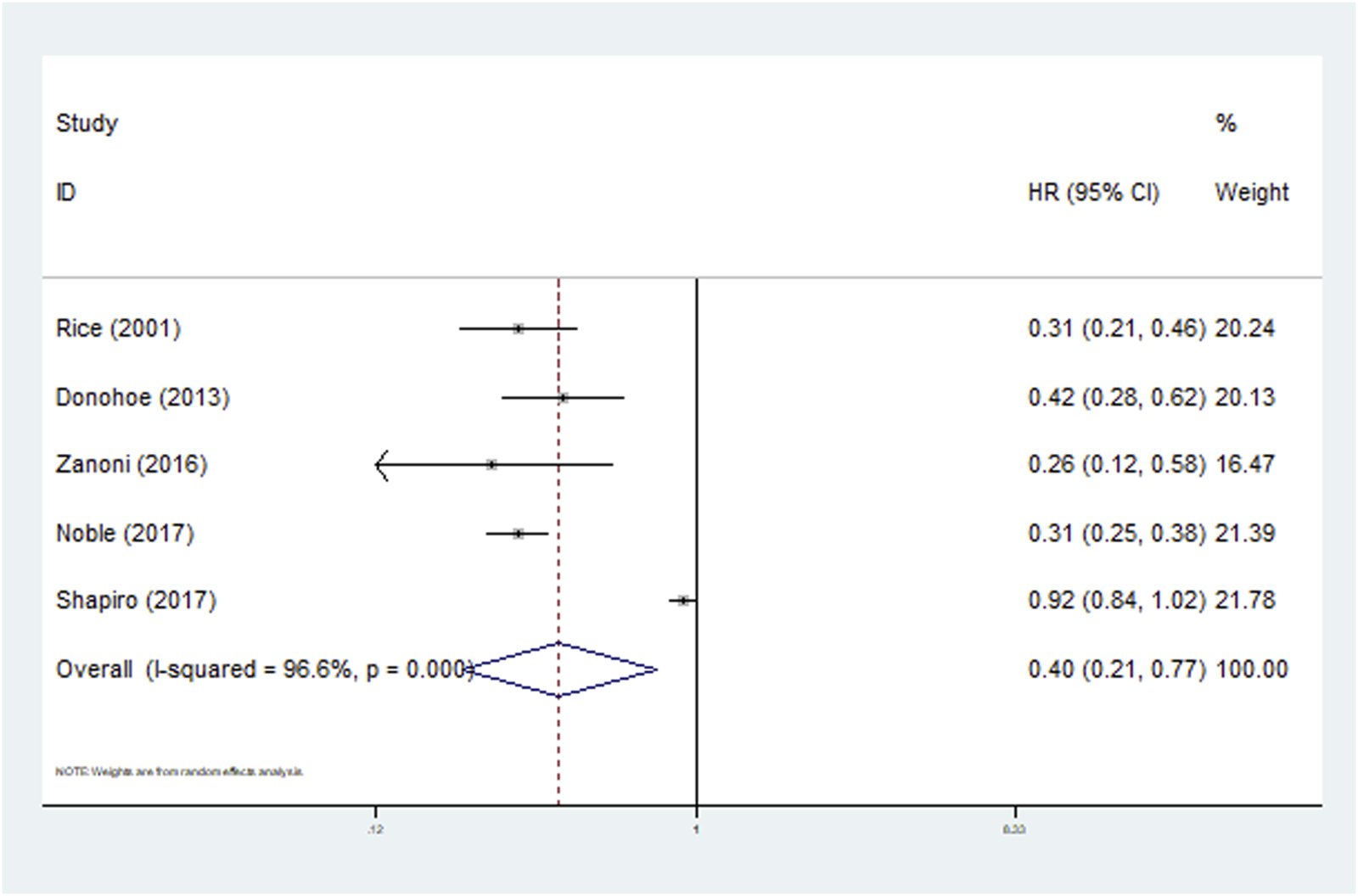
The association between N downstaging and overall survival in patients with esophageal cancer undergoing neoadjuvant therapy.
Furthermore, Zanoni et al. reported that N downstaging was also significantly associated with improved CSS (HR = 0.27, 95% CI: 0.12–0.60, P < 0.001) (Table 2).
Sensitivity analysis and publication bias
We conducted a sensitivity analysis on LNRG for OS. As shown in Figure 5, the pooled results of this meta-analysis were stable and reliable.
FIGURE 5
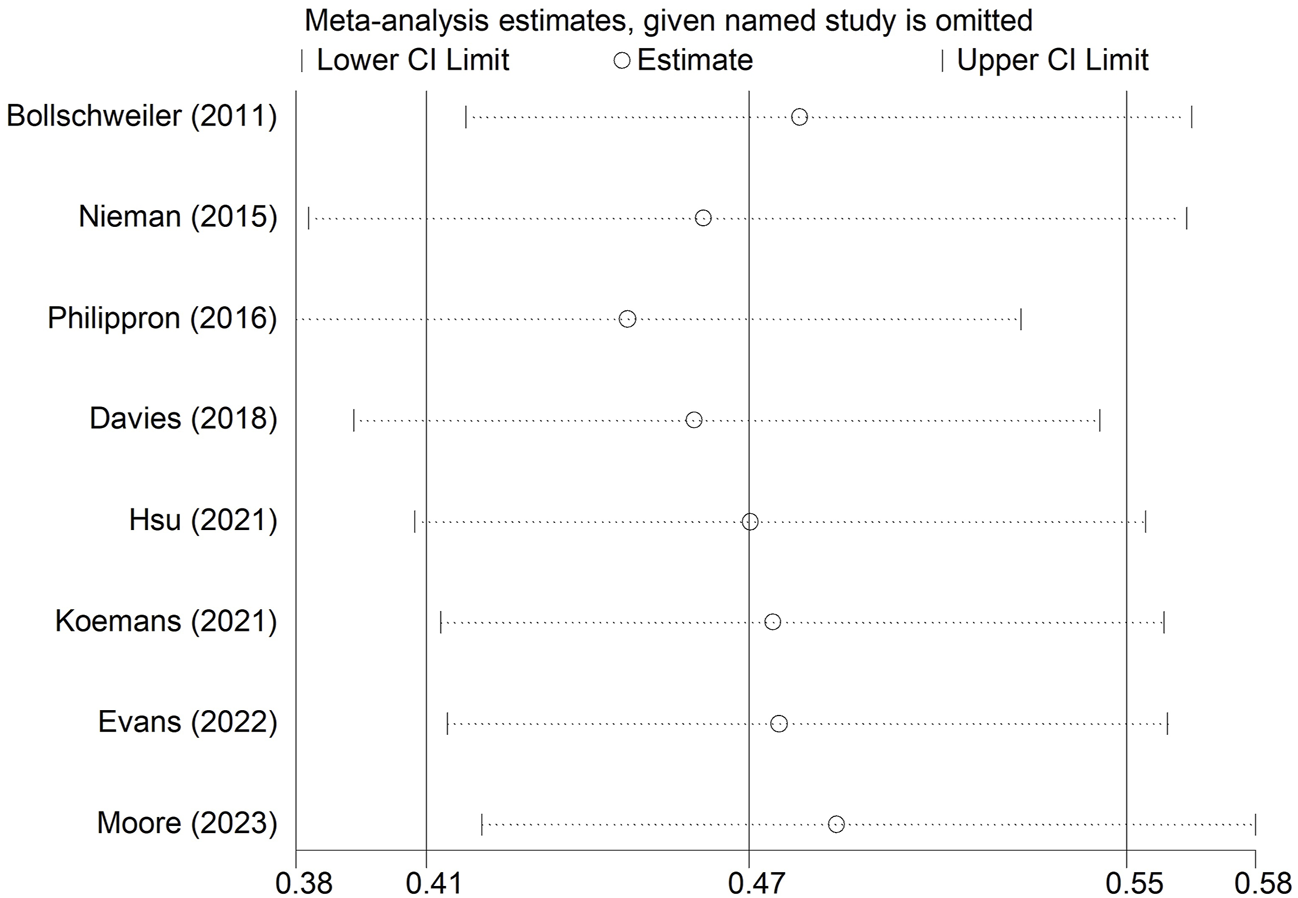
Sensitivity analysis for the association between lymph node regression grade and overall survival in patients with esophageal cancer undergoing neoadjuvant therapy.
Meanwhile, Begg’s funnel plot (Figure 6) and Egger’s test (P = 0.064) indicated that there was no obvious publication bias.
FIGURE 6
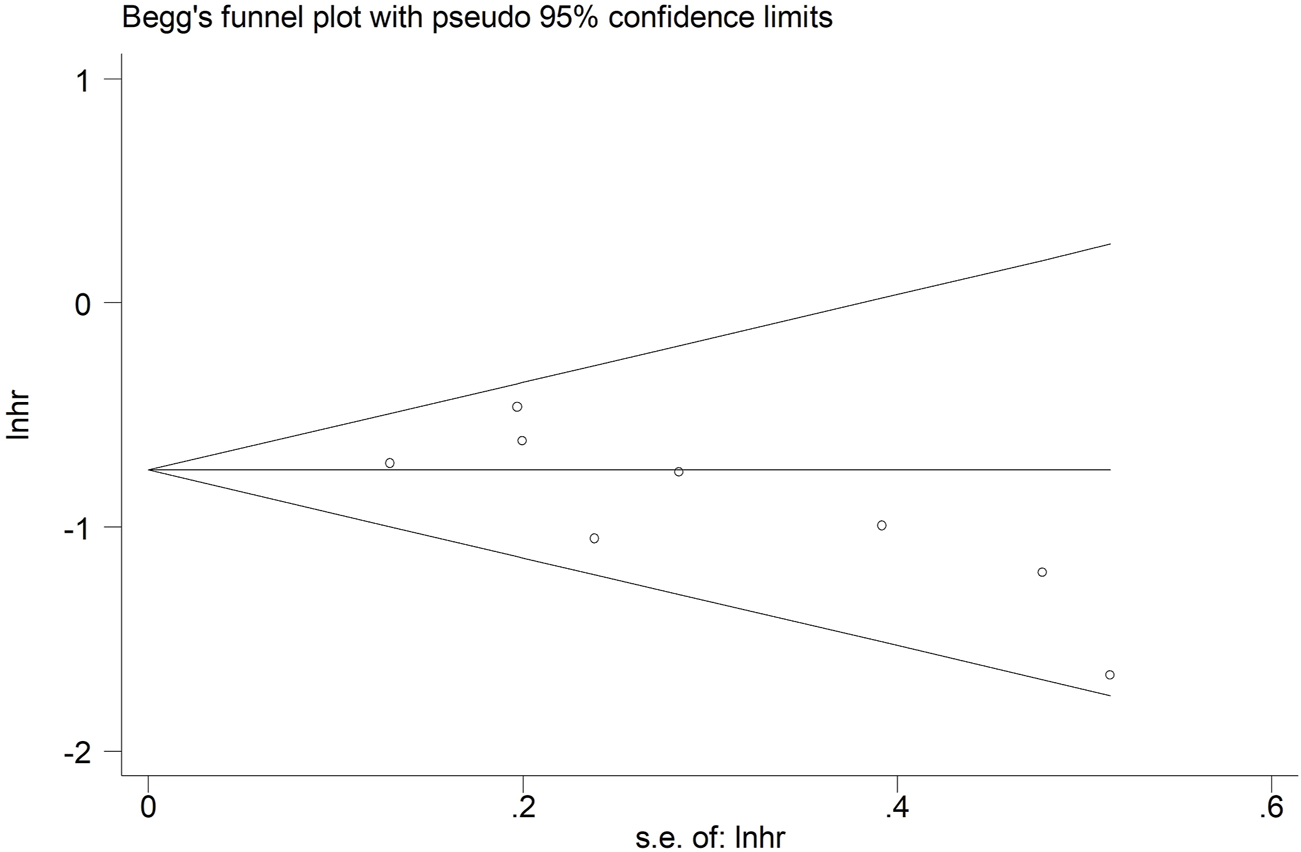
Begg’s funnel plot for the association between lymph node regression grade and overall survival in patients with esophageal cancer undergoing neoadjuvant therapy.
Discussion
According to this meta-analysis, LNR is significantly associated with long-term survival among patients with esophageal cancer who receive neoadjuvant therapy and patients with complete (or subtotal) LNR or (and) N downstaging are more likely to experience a better prognosis. Therefore, LNR should be carefully considered as a prognostic indicator in this group of patients.
Hagens et al. conducted a similar meta-analysis to explore the prognostic role of LNR in esophageal cancer [31]. They included eight studies, three of which assessed LNRG and five of which assessed N downstaging. According to their results, N downstaging was associated with improved survival (HR = 0.41, 95% CI: 0.22–0.77, P = 0.005). However, no significant association was observed between LNRG and survival (HR = 0.52, 95% CI: 0.26–1.06). Furthermore, a more detailed analysis was not performed in their meta-analysis [31]. Therefore, we conducted the current meta-analysis to further identify the prognostic relevance of LNR on survival in patients with esophageal cancer undergoing neoadjuvant therapy and to provide more evidence on the prognostic role of LNR in this group of patients.
The response to chemoradiotherapy may differ between lymph node metastases and primary lesions in esophageal cancer. First, cancer cells in primary lesions and metastases may exhibit different levels of differentiation [32]. Typically, cancer cells in primary lesions may be more differentiated, while those in metastases may be more unstable, heterogeneous, and may even contain more stem cell-like cells [33, 34]. This could lead to an increased resistance of metastases to chemotherapy [34]. Second, there may be differences in the microenvironment between metastatic lymph nodes and primary tumors, such as oxygen concentration, nutrient supply, and immune cell infiltration [35, 36]. These microenvironmental differences may affect the sensitivity of cancer cells to chemoradiotherapy [37]. Additionally, metastases may accumulate genetic mutations different from those in primary lesions, which may affect the sensitivity of cancer cells to anti-tumor drugs [38]. Specifically, metastases may develop resistance mutations to certain drugs [38]. While previous studies have often assessed the efficacy of neoadjuvant therapy based on changes in primary tumors, the evaluation of LNR status is also crucial in practice.
However, there is currently no standardized protocol for evaluating LNR, leading to variations in the grading systems used in the included studies, which needs to be further elucidated in future studies. At the same time, we believe it is also crucial to compare and assess the role of regression in primary tumors and lymph node metastases in predicting the prognosis of patients with esophageal cancer undergoing neoadjuvant therapy or to investigate the prognostic significance of joint evaluation of regression in primary tumors and lymph node metastases in response to neoadjuvant therapy. As reported by Yun et al., the combined evaluation of TRG and lymph node status demonstrated greater clinical value in predicting the prognosis of patients with esophageal cancer undergoing neoadjuvant therapy compared to TNM stage, TRG, and LNR [9]. Furthermore, whether LNR status contributes to guiding the formulation of postoperative adjuvant therapy or treatment strategies after recurrence in patients with esophageal cancer also needs to be explored.
Our meta-analysis has several limitations. First, the sample size was relatively small and all included studies were retrospective. Second, there were some confounding factors such as the surgical procedures, definition of LNRG, tumor stage, etc. Third, regarding LNRG, some studies included a small number of cN- patients, but did not specify whether these patients were excluded during the analysis, which may introduce a slight bias. Fourth, data from other countries were missing, more studies in other countries are still needed to verify our findings. Fifth, the neoadjuvant immunotherapy was not considered in all included studies. The association of LNR with prognosis in patients with esophageal cancer receiving neoadjuvant immunotherapy should be further investigated. Sixth, the optimal neoadjuvant chemotherapy or chemoradiotherapy regimen remains controversial, especially in patients with different clinical characteristics.
Conclusion
Overall, LNR could serve as a novel and reliable prognostic factor in patients with esophageal cancer undergoing neoadjuvant therapy and complete LNR and N downstaging predict better prognosis.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YW and CC conceived the study idea and designed the search strategy. PC and MC screened studies for eligibility and extracted data. PC and YB double appraised the results of studies for risk of bias. PC and MC undertook the data synthesis and analysis. PC and MC wrote the first drafts of the figures, tables, and appendices. PC and MC wrote the first draft of the manuscript. MC, YB, and GC interpreted the data analysis and critically revised the manuscript. YW and CC were the guarantors. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2024.1611844/full#supplementary-material
References
1.
Ju W Zheng R Zhang S Zeng H Sun K Wang S et al Cancer statistics in Chinese older people, 2022: current burden, time trends, and comparisons with the US, Japan, and the Republic of Korea. Sci China Life Sci (2023) 66(5):1079–91. 10.1007/s11427-022-2218-x
2.
Xia C Dong X Li H Cao M Sun D He S et al Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135(5):584–90. 10.1097/CM9.0000000000002108
3.
Yang W Niu Y Sun Y . Current neoadjuvant therapy for operable locally advanced esophageal cancer. Med Oncol (2023) 40(9):252. 10.1007/s12032-023-02097-4
4.
Liang Z Chen T Li W Lai H Li L Wu J et al Efficacy and safety of neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy in locally advanced esophageal cancer: an updated meta-analysis. Medicine (Baltimore) (2024) 103(3):e36785. 10.1097/MD.0000000000036785
5.
Tang H Wang H Fang Y Zhu JY Yin J Shen YX et al Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol (2023) 34(2):163–72. 10.1016/j.annonc.2022.10.508
6.
Eyck BM van Lanschot JJB Hulshof M van der Wilk BJ Shapiro J van Hagen P et al Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol (2021) 39(18):1995–2004. 10.1200/JCO.20.03614
7.
Li Q Liu T Ding Z . Neoadjuvant immunotherapy for resectable esophageal cancer: a review. Front Immunol (2022) 13:1051841. 10.3389/fimmu.2022.1051841
8.
Baksh K Prithviraj G Kim Y Hoffe S Shridhar R Coppola D et al Correlation between standardized uptake value in preneoadjuvant and postneoadjuvant chemoradiotherapy and tumor regression grade in patients with locally advanced esophageal cancer. Am J Clin Oncol (2018) 41(3):254–8. 10.1097/COC.0000000000000258
9.
Yun JK Kim Y Lee GD Choi S Kim YH Kim DK et al Tumor regression grade combined with lymph node status in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. Cancer Med (2022) 11(19):3623–32. 10.1002/cam4.4748
10.
Gu YM Lyu SM Shang QX Zhang HL Yang YS Wang WP et al Is tumor regression grade sufficient to predict survival in esophageal cancer with trimodal therapy? J Invest Surg (2022) 35(11-12):1818–23. 10.1080/08941939.2022.2127036
11.
Yasuda T Yano M Miyata H Yamasaki M Takiguchi S Fujiwara Y et al Prognostic significance of (18)F-fluorodeoxyglucose positron emission tomography (FDG-PET)-Positive lymph nodes following neoadjuvant chemotherapy and surgery for resectable thoracic esophageal squamous cell carcinoma. Ann Surg Oncol (2015) 22(8):2599–607. 10.1245/s10434-014-4299-9
12.
Zhang X Tan R Lam WC Yao L Wang X Cheng CW et al PRISMA (preferred reporting Items for systematic reviews and meta-analyses) extension for Chinese herbal medicines 2020 (PRISMA-CHM 2020). Am J Chin Med (2020) 48(6):1279–313. 10.1142/S0192415X20500639
13.
Wang Y Li J Chang S Dong Y Che G . Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: a systematic review and two meta-analyses based on four million cases. J Thorac Oncol : official Publ Int Assoc Study Lung Cancer (2021) 16(11):1893–908. 10.1016/j.jtho.2021.07.001
14.
Barili F Parolari A Kappetein PA Freemantle N . Statistical Primer: heterogeneity, random- or fixed-effects model analyses?Interact Cardiovasc Thorac Surg (2018) 27(3):317–21. 10.1093/icvts/ivy163
15.
Begg CB Mazumdar M . Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. 10.2307/2533446
16.
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) (1997) 315(7109):629–34. 10.1136/bmj.315.7109.629
17.
Rice TW Blackstone EH Adelstein DJ Zuccaro G Jr Vargo JJ Goldblum JR et al N1 esophageal carcinoma: the importance of staging and downstaging. J Thorac Cardiovasc Surg (2001) 121(3):454–64. 10.1067/mtc.2001.112470
18.
Bollschweiler E Hoelscher AH Metzger R Besch S Moenig SP Baldus SE et al Prognostic significance of a new grading system of lymph node morphology after neoadjuvant radiochemotherapy for esophageal. Ann Thorac Surg (2011) 92(6):2020–7. 10.1016/j.athoracsur.2011.06.091
19.
Donohoe CL O'Farrell NJ Grant T King S Clarke L Muldoon C et al Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg (2013) 258(5):784–92. discussion 792. 10.1097/SLA.0b013e3182a66588
20.
Nieman DR Peyre CG Watson TJ Cao W Lunt MD Lada MJ et al Neoadjuvant treatment response in negative nodes is an important prognosticator after esophagectomy. Ann Thorac Surg (2015) 99(1):277–83. 10.1016/j.athoracsur.2014.07.037
21.
Philippron A Bollschweiler E Kunikata A Plum P Schmidt C Favi F et al Prognostic relevance of lymph node regression after neoadjuvant chemoradiation for esophageal cancer. Semin Thorac Cardiovasc Surg (2016) 28(2):549–58. 10.1053/j.semtcvs.2016.04.003
22.
Zanoni A Verlato G Giacopuzzi S Motton M Casella F Weindelmayer J et al ypN0: does it matter how you get there? Nodal downstaging in esophageal cancer. Ann Surg Oncol (2016) 23(Suppl. 5):998–1004. 10.1245/s10434-016-5440-8
23.
Noble F Lloyd MA Turkington R Griffiths E O'Donovan M O'Neill JR et al Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br J Surg (2017) 104(13):1816–28. 10.1002/bjs.10627
24.
Shapiro J Biermann K van Klaveren D Offerhaus GJ Ten Kate FJ Meijer SL et al Prognostic value of pretreatment pathological tumor extent in patients treated with neoadjuvant chemoradiotherapy plus surgery for esophageal or junctional cancer. Ann Surg (2017) 265(2):356–62. 10.1097/SLA.0000000000001630
25.
Davies AR Myoteri D Zylstra J Baker CR Wulaningsih W Van Hemelrijck M et al Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br J Surg (2018) 105(12):1639–49. 10.1002/bjs.10900
26.
Hsu P-K Yeh Y-C Chien L-I Huang C-S Hsu H-S . Clinicopathological significance of pathologic complete lymph node regression after neoadjuvant chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg Oncol (2021) 28(4):2048–58. 10.1245/s10434-020-09363-z
27.
Koemans WJ Larue R Kloft M Ruisch JE Compter I Riedl RG et al Lymph node response to chemoradiotherapy in oesophageal cancer patients: relationship with radiotherapy fields. Esophagus (2021) 18(1):100–10. 10.1007/s10388-020-00777-y
28.
Evans RP Kamarajah SK Kunene V Zardo D Elshafie M Griffiths EA . Impact of neoadjuvant chemotherapy on nodal regression and survival in oesophageal adenocarcinoma. Eur J Surg Oncol (2022) 48(5):1001–10. 10.1016/j.ejso.2021.12.021
29.
Moore JL Green M Santaolalla A Deere H Evans RPT Elshafie M et al Pathologic lymph node regression after neoadjuvant chemotherapy predicts recurrence and survival in esophageal adenocarcinoma: a multicenter study in the United Kingdom. J Clin Oncol (2023) 41(28):4522–34. 10.1200/JCO.23.00139
30.
Yehan Z Ying L Peng G Zongyao H Chengmin Z Hong Y et al Prognostic significance of positive lymph node regression grade to neoadjuvant chemoradiation for esophageal squamous cell carcinoma. J Surg Oncol (2024) 129(4):708–17. 10.1002/jso.27555
31.
Hagens E Tukanova K Jamel S van Berge Henegouwen M Hanna GB Gisbertz S et al Prognostic relevance of lymph node regression on survival in esophageal cancer: a systematic review and meta-analysis. Dis Esophagus (2022) 35(1):doab021. 10.1093/dote/doab021
32.
Walter D Harter PN Battke F Winkelmann R Schneider M Holzer K et al Genetic heterogeneity of primary lesion and metastasis in small intestine neuroendocrine tumors. Sci Rep (2018) 8(1):3811. 10.1038/s41598-018-22115-0
33.
Choi JY Lee YS Shim DM Lee YK Seo SW . GNAQ knockdown promotes bone metastasis through epithelial-mesenchymal transition in lung cancer cells. Bone Joint Res (2021) 10(5):310–20. 10.1302/2046-3758.105.BJR-2020-0262.R3
34.
Fischer HP . Regression and therapy-resistance of primary liver tumors and liver metastases after regional chemotherapy and local tumor ablation. Pathologe (2005) 26(3):191–200. 10.1007/s00292-005-0749-2
35.
Popper H . Primary tumor and metastasis-sectioning the different steps of the metastatic cascade. Transl Lung Cancer Res (2020) 9(5):2277–300. 10.21037/tlcr-20-175
36.
Cacho-Díaz B García-Botello DR Wegman-Ostrosky T Reyes-Soto G Ortiz-Sánchez E Herrera-Montalvo LA . Tumor microenvironment differences between primary tumor and brain metastases. J Transl Med (2020) 18(1):1. 10.1186/s12967-019-02189-8
37.
Wood SL Pernemalm M Crosbie PA Whetton AD . The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev (2014) 40(4):558–66. 10.1016/j.ctrv.2013.10.001
38.
Xu CW Wang WX Wu MJ Zhu YC Zhuang W Lin G et al Comparison of the c-MET gene amplification between primary tumor and metastatic lymph nodes in non-small cell lung cancer. Thorac Cancer (2017) 8(5):417–22. 10.1111/1759-7714.12455
Summary
Keywords
lymph node regression, esophageal cancer, neoadjuvant therapy, survival, meta-analysis
Citation
Chen P, Chen M, Bu Y, Che G, Cheng C and Wang Y (2024) Prognostic role of lymph node regression in patients with esophageal cancer undergoing neoadjuvant therapy. Pathol. Oncol. Res. 30:1611844. doi: 10.3389/pore.2024.1611844
Received
22 May 2024
Accepted
19 September 2024
Published
11 October 2024
Volume
30 - 2024
Edited by
Judit Kocsis, Bács-Kiskun County Hospital, Hungary
Updates
Copyright
© 2024 Chen, Chen, Bu, Che, Cheng and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wang, wangyanxw@wchscu.cn; Chao Cheng, 505162410@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.