Abstract
Aim: This single institute prospective study aimed to evaluate the feasibility of LINAC-based stereotactic body radiotherapy (SBRT) in treating patients with early-stage non-small cell lung cancer (NSLSC). We focused on the survival data with the local and distant control profiles and the cancer- and non-cancer-specific survival. Treatment-related side effects were also collected and analyzed.
Methods: Patients with early-stage NSCLC between January 2018 and October 2021 were included in our prospective study; a total of 77 patients receiving LINAC-based SBRT were analyzed. All patients had pretreatment multidisciplinary tumor board decisions on SBRT. The average patient age was 68.8 years (median: 70 years, range: 52–82); 70 patients were in ECOG 0 status (91%), while seven patients were in ECOG 1-2 status (9%). 52% of the patients (40) had histologically verified NSCLC, and the other 48% were verified based on PETCT results. We applied the SBRT scheme 8 x 7.5 Gy for central tumors (74%) or 4 x 12 Gy for peripheral tumors (26%).
Results: The mean follow-up time was 25.4 months (median 23, range 18–50). The Kaplan-Meier estimation for overall survival in patients receiving LINAC-based SBRT was 41.67 months. Of the 77 patients treated by SBRT, death was reported for 17 patients (9 cases cancer-specific, 8 cases non-cancer specific reason). The mean local tumor control was 34.25 months (range 8.4–41), and the mean systemic control was 24.24 months (range 7–25). During the treatments, no Grade I-II were reported; in 30 cases, Grade I non-symptomatic treatment-related lung fibrosis and two asymptomatic rib fractures were reported.
Conclusion: In the treatment of early-stage NSCLC, LINAC-based SBRT can be a feasible alternative to surgery. Although we reported worse OS data in our patient cohort compared to the literature, the higher older average age and the initial worse general condition (ECOG1-2) in our patient cohort appear to be the reason for this difference. With the comparable local control and survival data and the favorable side effect profile, SBRT might be preferable over surgery in selected cases.
Introduction
Lung cancer in Europe represents a leading cause of cancer cases, with more than 312,000 newly diagnosed cases per year. Hungary is the leading European country in the incidence of lung cancer and has the highest mortality rate [1, 2]. Approximately 85% of all lung cancer incidences are non-small cell lung cancer (NSCLC) [3]. The gold standard curative protocol is surgery that mainly aims to reduce the disease progression, relieve the symptoms, and increase the overall survival (OS) if possible [4]. Many patients, however, are unable to tolerate thoracotomy due to comorbidities or personal preference. [5] The video-assisted thoracoscopic surgery (VATS) method was chosen as the treatment choice since it was reported to decrease the risk of complication after treatment and a higher 5-year survival rate than the open lobectomy method [5, 6]. Nevertheless, in some cases where resection is not possible due to the tumor location, functional status of the lung, or inoperable patients [7, 8], another treatment method should be evaluated and assessed.
LINAC-based stereotactic body radiation therapy (SBRT), which is an alternative to VATS, was found to be a choice of treatment, especially for elderly patients and those patients with more than one known disease [5]. This method was comparable to the VATS in previous clinical reports [5, 9–11].
SBRT is a state-of-the-art treatment method that uses radiation therapy to deliver high-dose ablative doses to tumors [11]. This method allows for successful tumor ablation with relatively high tumor control probability while keeping the surrounding tissues intact [7, 12]. Furthermore, this technology’s use and continuous improvement can improve results in potentially operable cases. Previously, limited studies have been compared between (SBRT) and (VATS) in terms of overall survival (OS), cancer-specific survival (CSS), loco-regional control (LCC), and disease-free survival (DFS). Therefore, this single institute prospective study aims to evaluate LINAC-based SBRT for NSCLC patients as 5 years of follow-up experience at Debrecen University regarding OS, CSS, LCC, and side effect profile.
Material and methods
Study population
This was a prospective mono-institutional study; the consent form was obtained from each patient. Patient data were collected and processed with the ethical permission of the Regional Research Ethics Committee. Demographic variables obtained from the electronic file database Clinic Center of the University of Debrecen (Debrecen, Hungary), called UD-MED, included age, gender, forced expiratory volume in 1 s to forced vital capacity ratio (FEV1/FVC%), and FEV1% predicted before treatment. All patients underwent pretreatment multidisciplinary tumor board before starting the SBRT at the Oncoradiology Clinic of the University of Debrecen (Debrecen, Hungary).
The average age was 68.8 years (median: 70 years, range: 52–82); 67 patients were in ECOG 0 status (87%), while ten patients were in ECOG 1-2 status (13%). 52% of the patients (40) had histologically verified NSCLC, and 48% were confirmed based on PET-CT with high FDG SUV (over the SUV value of 2.8). The mean FEV1 value was 1.06 (L), the mean FEV1 42%, the mean FVC 2.14 (L), and the mean FVC 62.69%.
Imaging
Chest CT examination is fundamental in tumor diagnostics and staging; a contrast-enhanced chest CT scan was used in all cases as a part of staging. Determining the patient’s respiratory function capacity was also crucial from the point of view of the operation and the execution of the radiation treatment. Before treatment, a bronchoscopy was performed in all cases. In cases where the bronchoscopy could not give proper histological information, a CT-guided needle biopsy was conducted where a better visualization of the tumor’s position was obtained when the tumor was smaller than 2 cm and when complications were more avoidable with such an examination. To decide oncological operability, enlarged lymph nodes detected on CT or PET-CT were valid only in conjunction with a positive histological examination. Suspicious patterns examined with CT can be supplemented with an FDG-PET examination, and in the case of non-small cell lung cancer, increased FDG uptake is observed. All patients receiving SBRT in our patient cohort had pretreatment FDG-PET scans within 2 weeks before the start of the treatment. A brain MRI was performed in all cases as a part of the staging.
Treatment procedures
For the 4D CT-based SBRT procedures, we used ELEKTA VERSA HD units with individual vacuum fixation systems and online 4D CBCT verification for each patient. Planning 4D CT was performed in the treatment position, with a slice thickness of 3 mm. No abdominal compression was applied during the process. Radiotherapy contouring and planning followed the department clinical protocol using the Pinnacle (Phillips, Netherlands) planning system (System version 16.2). To determine the exact gross tumor volume (GTV) and biological target volume (BTV), 4D planning CT-fused with FDG PET scans was used. Besides, the GTV and BTV internal target volume (ITV) was defined, using 4D CT information, to cover tumor movements. An additional 3–5 mm margin was used to generate the PTV. The mandatory OARs in planning were the lungs, heart, spinal cord, trachea, esophagus, chest wall, and great vessels per protocol. We applied the SBRT scheme of 8 × 7.5 Gy for central tumors (74%) or 4 × 12 Gy for peripheral tumors (26%). The treatments were delivered every other day (48-h shifts), with daily 4D CBCT verification and correction, if needed.
Data collection
Patients were followed up as follows: every 3 months for 2 years, every 6 months for another 3 years, and then annually. A medical history, physical examination, and chest CT were performed during the follow-up.
The primary endpoint of the study was local control (LC). We also examined systemic control, cancer-specific survival (CSS), and non-cancer-specific survival (NCSS). The analysis also focused on overall survival (OS) and treatment-related toxicity. OS was defined as the interval from the treatment date to any death or the last follow-up.
Statistical analysis
We used UD-MED, MEDSOL, and the Electronic Health Service Space (EESZT) for clinical data collection and analysis. The statistical analysis was performed with in-house-built Python scripts using the lifelines (v0.27.8) package [13].
Overall survival was estimated with the Kaplan Meier method (Figure 1). Risk estimation was performed using the Aalen-Johansen estimator to be able to assess risk in the different groups of patinets (Figure 2, Table 1).
FIGURE 1
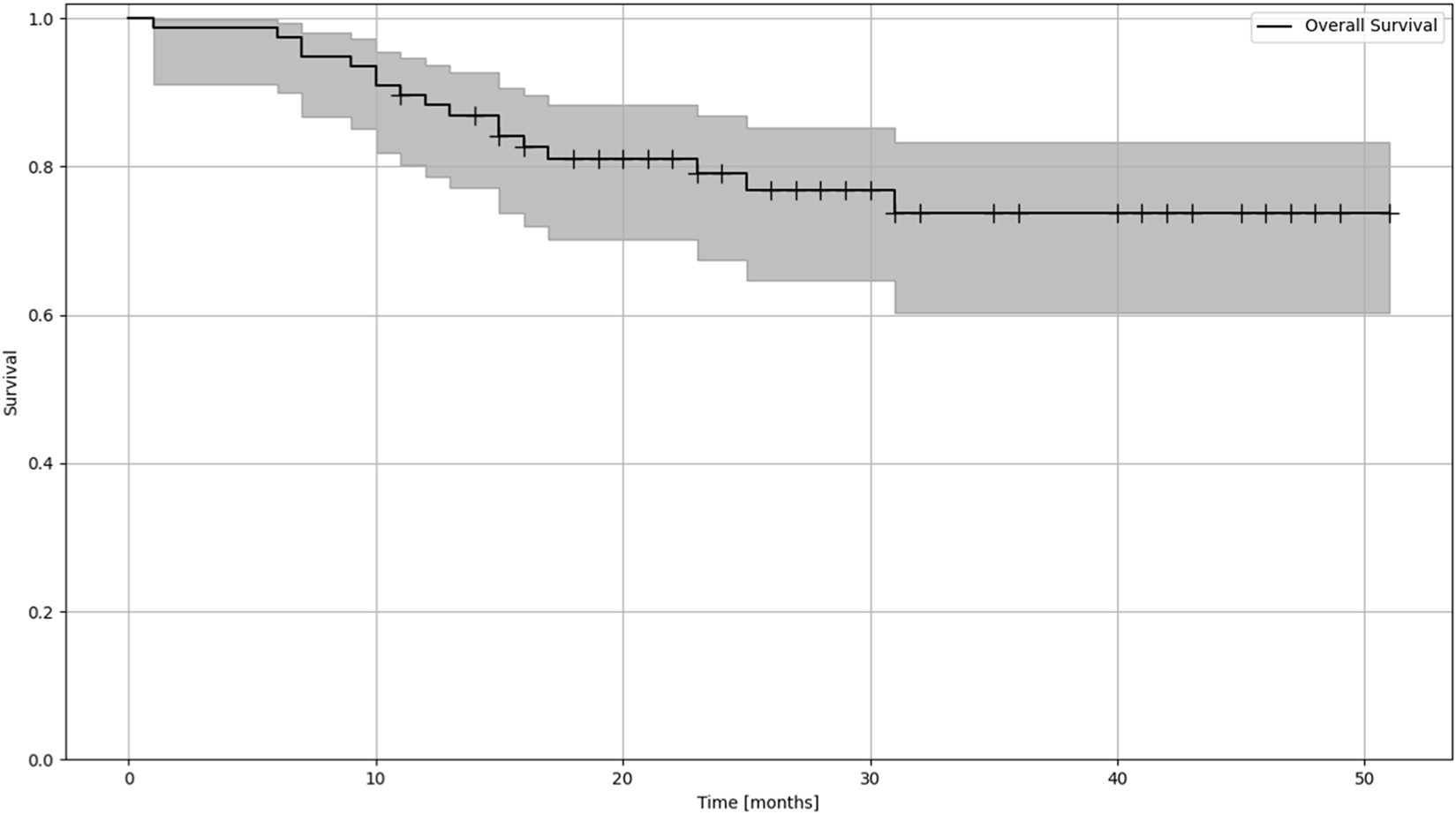
Kaplan-Meier estimation of OS (overall survival) of patients treated with lung SBRT.
FIGURE 2
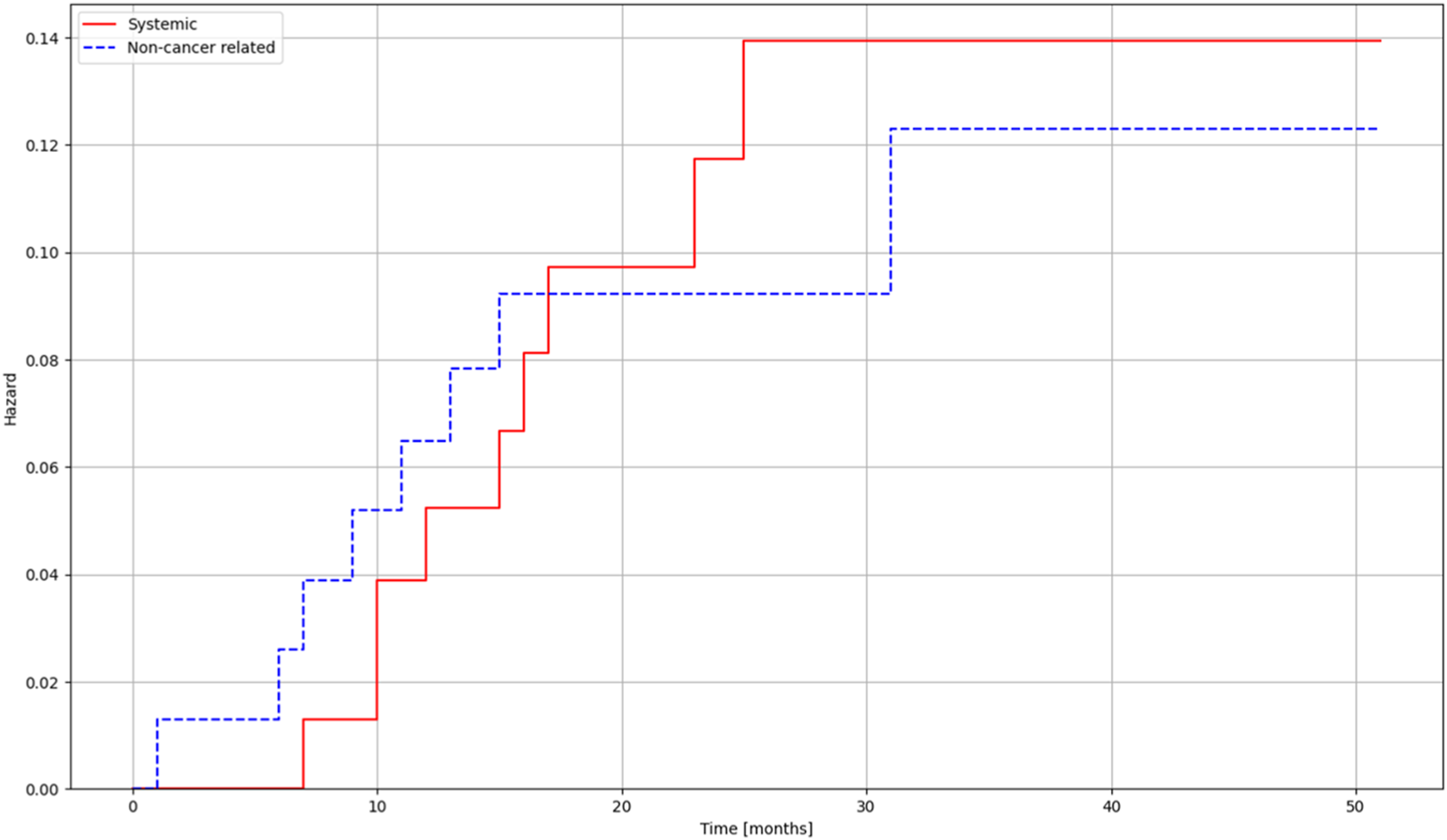
Risk estimates for deaths related to systemic progression and non-cancer-related deaths for patients treated with lung SBRT.
TABLE 1
| Times [months] | ||||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | |
| At risk | 77 | 72 | 46 | 25 | 12 | 3 |
| Censored | 0 | 0 | 17 | 36 | 48 | 57 |
| Events | 0 | 5 | 14 | 16 | 17 | 17 |
Aalen-Johansen risk estimate for cumulative risk of patients.
Results
The mean follow-up time was 25.4 months (median 23, range 18–50). The Kaplan-Meier estimation for overall survival in patients receiving LINAC-based SBRT was 41.67 months. Of the 77 patients treated by SBRT, death was reported for 17 patients (9 cases cancer-specific, 8 cases non-cancer specific reason). The mean local tumor control was 34.25 months (range 8.4–41), and the mean systemic control was 24.24 months (range 7–25) During the treatments, no Grade I-II side effect were reported; in 30 cases, Grade I non-symptomatic treatment-related lung fibrosis and two asymptomatic rib fractures were reported.
Discussion
The gold standard treatment option for early-stage NSCLC patients is still surgery, considered the first treatment of choice [14–16]. The state of art surgery is usually done with VATS to reduce patient encumbrance [17–19]. Considering the Overall Survival, Loco-regional Control, and Systemic Control data of the previously reported retrospective studies, SBRT is a full-fledged alternative to surgery for early-stage NSCLC patients [5, 20–22]. Besides the comparable local control and survival data, the main advantage of the SBRT is the favorable side effect profile and excellent tolerability, even in comorbid elderly patients [23]. Using SBRT also offers lower post‐treatment mortality [24]. In the literature, only a few studies focus on comparing SBRT and surgery because the comparison is made difficult by the patients’ different average ages and health statuses [5, 10–12, 25]. In our patient cohort, the higher average patient age, worse general condition, and initial respiratory functions are all reflected in the general patient selection process in the clinical decisions; the younger patients in good general condition are more frequently referred to surgery.
As in the previous studies, Dong et al., in their analysis of several studies, found that the results of SBRT were comparable to those of VATS. Thus, they reported that OS was comparable between the two groups with a statistically significant difference. They also reported comparable outcomes with no significant differences in terms of loco-regional failure, with 3- and 5-year rates of loco-regional failure for radiotherapy and surgery being 93.5% and 93.5% and 94.0% and 85.9%, respectively; furthermore, they reported that distal failure was comparable for both groups with no statistical significance between the groups [5].
In our prospective study, the SBRT-related loco-regional and systemic control results are comparable to the previously reported conventional surgery results in the literature [5, 7]. In the SBRT cohort, the hazard of death due to systemic progression barely exceeds the risk of dying from non-cancer-related reasons. We recorded no deaths due to local progression. For systemic control, we also examined the lymph node and distant metastases; SBRT showed promising results in terms of non-tumor-specific survival. The scope of SBRT indications should be expanded in the future, and further studies with more cases should be considered. Currently, the gold standard therapy of choice is still surgery [14, 16, 25], the advantage of which is the possibility of histological sampling.
It is important to note that in our prospective data analysis, some data were worse for patients who underwent SBRT, as reported in the literature; this can be explained by the fact that in our SBRT group, there were medically inoperable patients with worse respiratory function and with many comorbidities. Pulmonary function values were available before and after SBRT in some patients, and we observed improvement in FEV1 and FVC. The difference between recurrence and fibrosis can be difficult during follow-up, so monitoring the patient with PET-CT is essential. Another difficulty is the separation of metastases and secondary lung tumors.
Our patient cohort also noticed no acute side effects during SBRT. No late severe side effects were also described during oncological follow-up. This study aims to help clinicians the find the proper treatment of patients with early-stage NSCLC. The findings provided valuable information in answering these and other unresolved questions regarding SBRT.
Conclusion
In the treatment of early-stage NSCLC, LINAC-based SBRT can be a feasible alternative to surgery. We report moderately worse OS data in our patient cohort compared to the literature [5]. However, the difference in average age and the initial worse general condition (ECOG1-2) of our patient cohort can be an underlying reason. With the comparable local control and survival data and the favorable side effect profile, SBRT might be preferable over surgery in selected cases.
Statements
Data availability statement
The original contributions presented in the study are included in the article materials, further inquiries can be directed to the corresponding author. All the datasets are available to access.
Ethics statement
The studies involving human participants were reviewed and approved by the Regional Ethical Commitee of University of Debrecen, registration number: 599-2021. The patients provided written informed consent to participate in this study, approved by the ethical committee.
Author contributions
KT, MB, GK, EC, DS, and JP data collection, patient follow-up and data analysis, MS statistics, manuscript review, AK program leader, manuscript preparation. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
NSCLS, Non-small cell lung cancer; VATS, video-assisted thoracoscopic surgery; SBRT, Stereotactic body radiation therapy; LC, Local control; OS, Overall survival; CSS, cancer-specific survival; LCC, locoregional control; DFS, disease-free survival.
References
1.
LuCE Europe. Report on lung cancer: challenges in lung cancer in Europe. 6th Edn (2021). Available from: https://content/uploads/2017/10/LuCE-Report-final.pdf (Accessed November, 2021).
2.
Dyba T Randi G Bray F Martos C Giusti F Nicholson N et al The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer (2021) 157:308–47. 10.1016/j.ejca.2021.07.039
3.
Molina JR Yang P Cassivi SD Schild SE Adjei AA . Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc (2008) 83(5):584–94. 10.4065/83.5.584
4.
Bareschino MA Schettino C Rossi A Maione P Sacco PC Zeppa R et al Treatment of advanced non small cell lung cancer. J Thorac Dis (2011) 3(2):122–33. 10.3978/j.issn.2072-1439.2010.12.08
5.
Dong B Zhu X Shu Z Ji Y Lu F Wang J et al Video-assisted thoracoscopic lobectomy versus stereotactic body radiotherapy treatment for early-stage non-small cell lung cancer: a propensity score-matching analysis. Front Oncol (2020) 10:585709. 10.3389/fonc.2020.585709
6.
Cai Y Fu X Xu Q Sun W Zhang N . Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PLoS One (2013) 8(12):e82366. 10.1371/journal.pone.0082366
7.
Milano MT Kong FMS Movsas B . Stereotactic body radiotherapy as salvage treatment for recurrence of non-small cell lung cancer after prior surgery or radiotherapy. Transl Lung Cancer Res (2018) 8(1):78–87. 10.21037/tlcr.2018.08.15
8.
Ball D Mai GT Vinod S Babington S Ruben J Kron T et al Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol (2019) 20(4):494–503. 10.1016/S1470-2045(18)30896-9
9.
Boada M Guzmán R Montesinos M Libreros A Guirao A Sánchez-Lorente D et al Upstaging, centrality and survival in early stage non-small cell lung cancer video-assisted surgery. Lung Cancer (2019) 134:254–8. 10.1016/j.lungcan.2019.06.030
10.
Chi A Fang W Sun Y Wen S . Comparison of long-term survival of patients with early-stage non-small cell lung cancer after surgery vs stereotactic body radiotherapy. JAMA Netw Open (2019) 2(11):e1915724. 10.1001/jamanetworkopen.2019.15724
11.
Hamaji M Chen F Matsuo Y Kawaguchi A Morita S Ueki N et al Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg (2015) 99(4):1122–9. 10.1016/j.athoracsur.2014.11.009
12.
Tandberg DJ Tong BC Ackerson BG Kelsey CR . Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: a comprehensive review. Cancer (2018) 124(4):667–78. 10.1002/cncr.31196
13.
Davidson-Pilon C . Lifelines: survival analysis in Python. J Open Source Softw (2019) 4(40):1317. 10.21105/joss.01317
14.
Zarogoulidis K Zarogoulidis P Darwiche K Boutsikou E Machairiotis N Tsakiridis K et al Treatment of non-small cell lung cancer (NSCLC). J Thorac Dis (2013) 5(4):2–9. 10.3978/j.issn.2072-1439.2013.07.10
15.
Darling GE Decker PA Ballman K Malthaner RA Inculet RI Jones DR et al Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest (2011) 139(5):1124–9. 10.1378/chest.10-0859
16.
Howington JA Blum MG Chang AC Balekian AA Murthy SC . Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest (2013) 143(5 Suppl):e278S–e313S. 10.1378/chest.12-2359
17.
Detillon DDEMA Veen EJ . Postoperative outcome after pulmonary surgery for non-small cell lung cancer in elderly patients. Ann Thorac Surg (2018) 105(1):287–93. 10.1016/j.athoracsur.2017.07.032
18.
Burt BM Kosinski AS Shrager JB Onaitis MW Weigel T . Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg (2014) 148(1):19–28. discussion 28-29.e1. 10.1016/j.jtcvs.2014.03.007
19.
Nwogu CE Pang H Gu L Wang X Richards WG Veit LJ et al VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg (2015) 99(2):399–405. 10.1016/j.athoracsur.2014.09.018
20.
Prezzano KM Ma SJ Hermann GM Rivers CI Gomez-Suescun JA Singh AK . Stereotactic body radiation therapy for non-small cell lung cancer: a review. World J Clin Oncol (2019) 10(1):14–27. 10.5306/wjco.v10.i1.14
21.
Vansteenkiste J Crinò L Dooms C Douillard JY Faivre-Finn C Lim E et al 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol (2014) 25(8):1462–74. 10.1093/annonc/mdu089
22.
Grills IS Mangona VS Welsh R Chmielewski G McInerney E Martin S et al Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol (2010) 28(6):928–35. 10.1200/JCO.2009.25.0928
23.
Chua GWY Chua KLM . Which patients benefit most from stereotactic body radiotherapy or surgery in medically operable non-small cell lung cancer? An in-depth look at patient characteristics on both sides of the debate. Thorac Cancer (2019) 10(10):1857–67. 10.1111/1759-7714.13160
24.
Stokes WA Bronsert MR Meguid RA Blum MG Jones BL Koshy M et al Post-treatment mortality after surgery and stereotactic body radiotherapy for early-stage non-small-cell lung cancer. J Clin Oncol (2018) 36(7):642–51. 10.1200/JCO.2017.75.6536
25.
Mayne NR Darling AJ Raman V Patel DC Liou DZ D'Amico TA et al Stereotactic body radiotherapy versus delayed surgery for early-stage non-small-cell lung cancer. Ann Surg (2020) 272(6):925–9. 10.1097/SLA.0000000000004363
Summary
Keywords
VATS, NSCLC, SBRT, LINAC-based SBRT, early stage
Citation
Kovács Á, Trási K, Barabás M, Gál K, Csiki E, Sipos D, Papp J and Simon M (2024) LINAC-based SBRT in treating early-stage NSCLC patients—single institution experience and survival data analysis. Pathol. Oncol. Res. 30:1611589. doi: 10.3389/pore.2024.1611589
Received
13 November 2023
Accepted
25 January 2024
Published
13 February 2024
Volume
30 - 2024
Edited by
Nora Bittner, National Koranyi Institute of TB and Pulmonology, Hungary
Updates
Copyright
© 2024 Kovács, Trási, Barabás, Gál, Csiki, Sipos, Papp and Simon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Árpád Kovács, kovacsarpad@med.unideb.hu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.