Abstract
The vast majority of hormone positive and HER2 negative advanced breast cancers can be controlled well by endocrine therapy combined with the groundbreaking use of CDK4/6 inhibitors in the metastatic first-line setting. Approximately 50%–60% of these patients have “bone-only” metastatic disease. In oligometastatic cases or if a certain number of uncontrolled lesions develop during the aforementioned therapy, ablative radiotherapy can be delivered or, in symptomatic cases, urgent irradiation is needed with palliative intent. To achieve the most effective results, parallel with good quality of life, the timing of radiotherapy must be determined precisely, taking into account that different cell cycles are involved during different treatment modalities; therefore, optimization of treatment schedules ensures longer and safer post-progression overall survival. The key question is whether the two treatment modalities are safe concurrently or whether they should be administered separately, and if so, what is the optimal sequence and why? This manuscript aims to answer this important question, with a focus on quality of life. Existing publications focus on safety and toxicity profiles, and efficacy is detailed only tangentially and minimally.
Introduction
Breast cancer is the most frequently diagnosed malignancy and the leading cause of cancer-related mortality in women, worldwide [1]. The vast majority, approximately 75% of breast cancer patients, belongs to hormone-positive subtypes, with a relatively good prognosis [2]; thus, endocrine therapy is a highly effective treatment for these patients. Approximately 30% of women with an early-stage breast cancer diagnosis develop metastasis [3]. The metastatic site depends on the pathological subtype of the primary tumor, but in breast cancer, 30%–60% have metastases in the bone, 21%–32% in the lung, 15%–32% in the liver, and 4%–10% in the brain based on the SEER database [4]. Only 13% of primary breast cancer patients who developed metastasis in the bone survived >5 years in a Danish population-based cohort study [5].
New targeted drugs had to be developed to treat such cases. In cases of hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer, based on risk factors, selective cyclin-dependent kinase 4/6 (CDK4/6) inhibitors are widely used to postpone the need for chemotherapy. Up until now, three CDK4/6 inhibitors have been approved for the treatment of HR-positive advanced or metastatic breast cancer [6]. Palbociclib was the first authorized by the European Medicine Agency in November 2016 based on PALOMA studies [7], followed by ribociclib in August 2017 based on MONALEESA studies [8] and abemaciclib in September 2018 based on MONARCH studies [9]. These drugs are generally used in combination with hormone replacement therapies, considering current menopausal status, although abemaciclib was also approved as monotherapy. The latter was also approved for node-positive, high recurrence risk, Ki67 score ≥20% HR+ luminal B type breast cancer patients in adjuvant settings in October 2021 based on a monarchE study [10]. Beyond these, there are further drugs on the horizon. Dalpiciclib, which has good penetration of the blood-brain barrier, based on a DAWNA-1 randomized phase 3 trial [11], was conducted only in Chinese subjects, so its performance in other populations is still an open question. GLR2007 is also a promising new CDK4/6 inhibitor [12] that is effective in smaller concentrations compared with abemaciclib, which also has proper blood-brain barrier permeation. This suggests fewer adverse events and more effective treatment in cases of central nervous system metastasis. Lerociclib accumulates better in xenografts in preclinical studies; thus, fewer hematological and gastrointestinal side effects are presumed to be observed. Trilaciclib is also a promising new drug, which differs from all the above-mentioned drugs due to its intravenous administration route [13].
Approximately 50%–60% of hormone-positive and HER2-negative advanced breast cancer patients have “bone-only” disease [14]. Depending on the number, site, and size of metastasis, radiotherapy is often suggested as a local treatment modality to dominate symptoms or block tumor growth. Radiation can be delivered with palliative intent, e.g., pain relief, or even with local ablative intent to destroy certain small-size metastasis, mainly in oligometastatic settings. The main question is whether the two treatment modalities are safe concurrently or should be administered separately, and if so, what is the optimal sequence and why? This study investigates this pressing question from a molecular biology point of view and how we can implement the results, based on preclinical studies [15–17], into daily clinical practice.
Molecular biology aspects of certain breast cancer treatments
Radiotherapy
More than 100 years passed between the first breast radical mastectomy being performed and the development of targeted therapies in breast cancer treatment [18]. As we understand more about the biology of cancer, more sophisticated treatment methodologies will become available. Since there are still gaps in our knowledge, we are only able to achieve partial success with our commonly available (surgery, radiotherapy, chemotherapy) and more frequently used (hormone, immunotherapy, targeted therapy) tools.
Radiotherapy (RT) is widely used in cancer treatment. The first utilization of RT was in the early 20th century. RT treatment modalities—from superficial X-ray to ultra-high dose rate FLASH radiotherapy—have changed substantially [19], yet the main radiobiology concept remains the same. Ionizing radiation has direct and indirect effects by producing water radicals on DNA chains. Both causes mainly double-strand break and single-strand lesions, respectively [20, 21]. When DNA suffers irreparable damage, a damage signal cascade starts involving ATM, Chk2, and p53 proteins and stops cell cycles at the G1/S phase [22]. When DNA suffers single-strand break DNA damage, another pathway starts involving ATR, and Chk1, and this stops the cell cycle at the G2/M checkpoint [23]. Cancer cells often lose normal p53 function and cannot stop at the G1/S checkpoint. In this case, cells can only use G2/M checkpoints, while normal cells can stop and repair DNA at the G1/S phase.
Cells have a different sensitivity to ionization exposure that leads to different radiation-induced cell death [24]. The most sensitive phase of radiotherapy is mitosis.
Radiotherapy is not target selective, it acts on rapidly growing cancer and normal cells, too, but we can modulate its fraction, energy, and direction. Novel technological advances, such as intensity-modulated and image-guided radiotherapy were improved to deliver therapeutic doses and boost the gross tumor volume (GTV), thus increasing the difference between tumor control probability and normal tissue complications. Other advances, such as stereotactic body radiotherapy (SBRT), have enhanced dose conformity to substantially limit treatment margins, so a critical ablative dose of irradiation can be delivered to the tumor in one (radiosurgery) or in several fractions [25].
CDK4/6 inhibitors
Cell cycle is a highly ordered system, driven by cyclin-dependent kinases (CDKs). Cyclin-dependent kinase inhibitors target overactive CDKs. CDK4 and CDK6 have two key functions. In a complex with cyclin-D, CDKs phosphorylate retinoblastoma (Rb) protein, a tumor suppressor protein that activates E2F transcription factor, and sequestering cell cycle inhibitors p21Cip1 and p27Kip1 proteins, which leads to activation of cyclin-E dependent CDK2 containing complexes [26]. Cyclin-D has three isoforms: cyclin-D1, cyclin-D2, and cyclin-D3. Isoforms have different lengths; cyclin-D1 is 295 (UniProt:P24385), cyclin-D2 is 289 (UniProt:P30281), and cyclin-D3 is 292 (UniProt:P30279) amino acids long. The phosphorylation site for ubiquitination is also different in cyclin-D1 at Thr-286, in cyclin-D2 at Thr-280, and in cyclin-D3 at Thr-283 position. These isoforms form a heterodimer complex with CDK4/6 and in this complex can phosphorylate and, thus, inactivate Rb.
CDK4/6 inhibitors bind to the ATP domain of CDK4/6 but have different cyclin-D targets. Palbociclib inhibits D1-CDK4, D2-CDK6, and D3-CDK4. Ribociclib attacks D1-CDK4 and D3-CDK6. Abemaciclib targets D1-CDK4 and D1-CDK6 [17].
Mechanistically, pharmacological CDK4/6 inhibition prevents the phosphorylation of downstream cell cycle proteins, such as Rb, that control cell cycle progression through the G1/S checkpoint [27]. Inhibition of CDK4 and CDK6 induces cell cycle arrest at the G1/S checkpoint.
In breast cancer, active cyclin-D in a complex with CDK4/6 is considered to play a high-impact role in estrogen-driven cell proliferation [28, 29]. In clinical practice, novel FDA-approved CDK4/6 inhibitors are used in breast cancer treatment [30].
A combination of common and new treatments is recommended in clinical guidelines. However, the ideal treatment combination and timing to reach their synergistic effect are not yet clear. CDK4/6 inhibitors and other DNA-damaging therapies, like certain chemotherapies or radiotherapy, need proper therapeutic order as they target different cell cycle phases. If CDK4/6 inhibitors arrest the cycle in the G1/S or G0/G1 phase, it prevents the cells from entering the subsequent phase. An antagonistic effect occurs as cell cycles do not enter into their next phases, so radiotherapy cannot develop its complete effect [13]. If irradiation is required and CDK4/6 inhibitors should be suspended, the elimination time of different drugs must be taken into account (Figure 1).
FIGURE 1
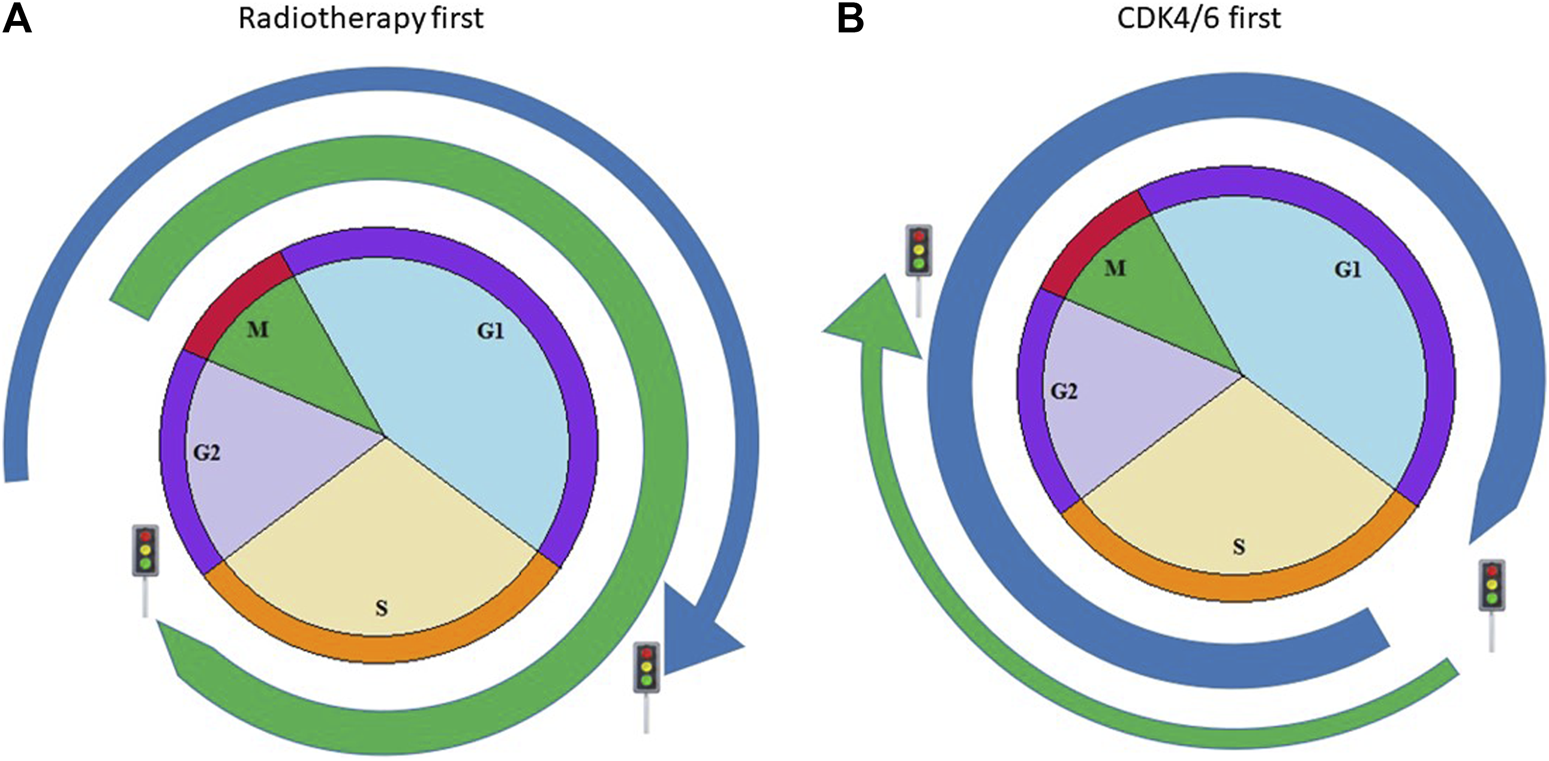
Possible combination of CDK4/6 inhibitors with radiotherapy. Blue arrow simulates the effect of CDK4/6 inhibitors according to cell cycles, green arrow shows the acting point of radiotherapy in cell cycle. (A) Figure shows the case when radiotherapy is delivered before CKD4/6 inhibitors and acts in the M phase, resulting in more cancer cell deaths (B) Figure shows the case when CDK4/6 inhibitors block cancerous cells in an earlier phase; therefore, tumorous cells cannot reach the M phase, which is the most sensitive phase to radiotherapy.
Pharmacokinetics of CDK4/6 inhibitors are slightly different. Their metabolism is through CYP3A4 enzyme, and palbociclib also interacts with SULT2A1. Their half-life times are also different: 24–34 h of palbociclib, 30–55 h of ribociclib, and 17–38 h of abemaciclib. Within 3 days, they half, suggesting a 1 week drug-free period before irradiation [12].
CDK4/6 and radiotherapy in vitro/in vivo studies
Pesch et al. tested the effect of CDKi alone and in combination with RT on ER+ breast cancer cell lines and in mouse xenografts. They conclude that in radiosensitized cell lines, CDKi suppresses cell cycle signaling and changes the DNA repair response. In mouse xenograft models, concurrent administration of CDKi and RT suppressed the tumor growth and prolonged tumor doubling time compared to controls that received monotherapies [31]. In a recent study, Klapp et al. tested a sequenced RT and CDKi treatment and measured its optimal schedule through cellular senescence. They demonstrated that the RT-first approach induced an increased level of cellular senescence in cell lines and a mouse model, respectively [32].
The bone-only metastatic breast cancer treatment cannot be modeled in preclinical cell culture or in animal experiments as it is special circumstance.
CDK4/6 and radiotherapy clinical trials
The main difference between the two modalities is that radiotherapy stimulates cells toward certain cell death, while CDK4/6 inhibitors block cell cycle progression at the G1/S checkpoint. Pretreated cells by CDK4/6 inhibitors theoretically are less sensitive to radiotherapy as they are blocked at G1/S.
Few trials have investigated the effects of CDK4/6 inhibitors alone or in combination with radiotherapy according to ClinicalTrials.gov. There are only five ongoing or recruiting clinical trials investigating CDK4/6 inhibitors with radiation therapy in breast cancer with bone metastases (Table 1).
TABLE 1
| Trial number/name | Phase | Enrollment criteria | Fractionation | Drug | Association RT/CDK4/6inhibitors | Treatment sequence | Recruitment status | Planned enrollment | Actual enrollment |
|---|---|---|---|---|---|---|---|---|---|
| NCT03691493 | Phase 2 | de novo bone metastasis | Conventionally | Palbociclib | Concurrent | - | Active, not recruiting | 42 | 38 |
| ASPIRE | |||||||||
| NCT05334459 | Phase 1 | de novo oligometastatic | Conventionally | CDK 4/6i | Sequential | N.A. | Recruiting | 40 | N.A. |
| ISTMET | |||||||||
| NCT03870919 | N.A. | de novo bone-only | Conventionally | Palbociclib | Sequential | RT after 6 cycles systemic treatment | Recruiting | 200 | N.A. |
| PALATINE | |||||||||
| NCT03750396 | Phase 2 | de novo metastatic | Mainly SBRT | CDK 4/6i or mTORi | N.A. | N.A. | Recruiting | 110 | N.A. |
| CLEAR | |||||||||
| ACTRN12620001212943 | Phase 2 | de novo oligometastatic | SBRT | CDK4/6i | Sequential | Radiotherapy in CDK4/6 pause | Recruiting | 32 | N.A. |
| AVATAR |
Ongoing clinical trials of CDK4/6 inhibitors in combination with radiotherapy in metastatic breast cancer.
In an NCT03691493 phase 2 trial, CDK4/6 inhibitor (palbociclib) in combination with a nonsteroidal aromatase inhibitor and radiation therapy was used; in an NCT03870919 trial, only a locoregional treatment with palbociclib was used; and in NCT04923542 phase 1 & 2 trials, abemaciclib in combination with endocrine therapy and stereotactic radiosurgery was used. According to the currently recruiting AVATAR phase 2 study protocol, CDK4/6 inhibitors must be stopped at least 3 days prior to SRT and allowed to continue 3 days following STR completion. The authors also declare that a week off after CDK4/6 inhibitor treatment is optimal if feasible [33].
Besides breast cancer, there are trials investigating the effects of CDK4/6 inhibitors in prostate cancer, head and neck cancer, and gliomas.
Toxicity of radiotherapy and CDK 4/6 inhibitor therapies
Meattini et al. reported preliminary experience with five metastatic breast cancer patients who received concomitant ribliciclib and palliative RT. Two of five patients had G3-4 adverse events (neutropenia, vomiting, and diarrhea) [34]. David et al. reported five cases with G2-5 adverse events (pneumonitis, dermatitis, oesophagitis) during palbociclib and RT concurrent or separate treatment [35]. A safety and feasibility study conducted on 288 ER+ advanced breast cancer patients used RT and CDKi sequentially and concurrently. This retrospective study mainly focused on severe adverse events. They found that neutropenia was more frequent in a concurrent treatment arm [36]. Based on these studies, concurrent treatment has the potential to result in more adverse events.
The concurrent use of CDK4/6 inhibitors and radiotherapy may indeed lead to an increased risk of side effects. Both CDK4/6 inhibitors and radiotherapy can individually cause side effects, and their combination can potentially amplify these. However, it is important to note that the specific side effects and their severity can vary depending on the individual patient, specific drugs, and radiation protocols used. When CDK4/6 inhibitors and radiotherapy are used concurrently, there can be overlapping or additive effects on the body, potentially leading to increased side effects.
Translational implications/clinical applications
The optimal timing of radiotherapy and CDK4/6 inhibitor plus endocrine partner therapy administration is translational implication; therefore, it could be a major benefit from the view of clinical application [16].
In drug registration clinical trials [37–40], irradiation was allowed during CDK4/6 inhibitor therapy. In a PALOMA trial, only 1.9% of the randomized subjects received radiotherapy [7, 41, 42] and it was highlighted that in cases of central nervous system metastasis treated with irradiation, CDK4/6 inhibitors were prohibited.
The more metastatic treatment lines we indicate, the shorter the progression-free survival time. Therefore, it is very important to maintain effective treatment albeit it could not dominate a certain number (less than three) lesions. Thus, with the implementation of impressive local ablative treatment, there is no need for drug switching. This is a paradigm shift in oncology: we understand that malignancies have heterologous clone populations and just those can skip from treatment control whose have acquired resistance. Therefore, except for these lines, properly chosen therapy can block sensitive clones and can be continued safely and undisturbed. Uncontrolled lines can be treated by local ablative treatment methods. If the required result appears on re-staging, i.e., the blockade or destruction of the so-far uncontrolled tumor lesions, the post-progression overall survival time can be prolonged. Significant benefits and overall survival improvement are expected. In such a case, there is no need to modify further therapeutic lines; therefore, good quality of life can be maintained by postponing new drug introduction. Patients can gain time, with a considerably improved and extended lifespan.
Moreover, the latest studies have revealed that CDK4/6 inhibitors may stimulate the immune system, namely, CD8 T-cell immune memory cells, interact with the tumor microenvironment, and have antitumor immunity effects [15, 43].
Radiotherapy is also an immunogenic treatment modality as it causes stress-driven regulated cell death; nowadays, so-called immunogenic cell death is an adaptive immune response [15, 21, 44]. It sensitizes the microenvironment before immune therapies [45], so the primary given radiotherapy may not just be synergistic but may even be a supra-additive effective treatment, taking into account that CDK4/6 inhibitors also affect the immune response.
Discussion
The toxicity profile of concomitant CDK4/6 inhibitor therapy with irradiation is still unclear, though there are few preclinical studies [30, 45, 46] targeting this question. There are a few that conclude the radiosensitization effects of CDK4/6 inhibitors, although not in every tumor type, mainly in HPV-positive, high-mitotic activity, and head and neck squamous cell cancer, whose biological features are different from relatively slow-growing hormone receptor-positive breast cancers. These studies speculate that the CDK4/6 inhibitor probably blocks cell cycle progression into the radioresistant S phase, but these conclusions have not been substantiated in daily clinical practice [47].
Following exhaustive publication searches, few case series and reports were found to be available, involving few subjects and without control groups, focusing mainly on toxicity profiles with controversial results. Furthermore, the efficacy results of concomitant CDK4/6 inhibitor therapy with irradiation are not detailed. A recently published clinical investigation within a multi-institutional safety and toxicity study reported higher toxicity rates when these two modalities were administered concurrently. According to this clinical investigation, safety and toxicity results appear to be clear. Therefore, the question remains as to which administration schedule is more favorable.
The sequence and timing of different therapies are crucial to reach optimal antitumor effects [16]. The main difference between radiotherapy and CDK4/6 inhibitors is their cancer control: radiotherapy drives cells to certain cell death, while CDK4/6 inhibitors block the cell cycle at the G1/S checkpoint. Pre-treated cells by CDK4/6 inhibitors are theoretically less sensitive to radiotherapy as they block at G1/S. In case of proper order palliative effect is more favorable; therefore, a longer progression-free survival and a better quality of life can be achieved.
The authors suggest the following order in those cases when the delivery of radiotherapy and CDK4/6 inhibitor treatment are both indicated.
If de novo diagnosed metastatic breast cancer requires CDK4/6 inhibitor therapy, start with irradiation and then administer CDK4/6 inhibitors and endocrine replacement therapy [48].
When a patient is under treatment with CDK4/6 inhibitor, there are two options depending on the aim and intent of high-energy radiotherapy. In the first case, if palliative ultra-hypofractionated radiation is planned due to severe pain relief, or if there is a risk of fracture or compression caused by bone metastasis that cannot be treated with local ablative dose, 8Gy in a single fraction can be scheduled. Radiotherapy can be given at the end of the drug therapeutic gap (21/7 days schema) in the case of palbociclib and ribociclib. Nota bene, the abemaciclib schedule is continuous; therefore, it can be suspended and radiotherapy can be implemented a week later.
In the second case, in oligometastatic disease or in certain non-responder foci or few (less than three) newer lesions, where treatment with a curative dose is planned, even with stereotactic body radiation or intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) with simultaneous integrated boost (SIB), the radiation can be initiated a week after the last pastille intake of CDK4/6 inhibitor. Following the suspension of CDK4/6 inhibitors and completion of radiotherapy, it can be re-administered safely and allowed to continue if restaging radio-biological images show no progression. The re-evaluation of radiological images has to be conducted using the same method used for diagnosis and must be scheduled 6–8 weeks following the completion of radiotherapy. In the case of metabolic information that can be measured on PET/CT, the maximum standardized uptake value (SUVmax) can show the activity changes (hopefully decay) of the tumor.
Conclusion
After publications and clinical trials were investigated, from molecular biology and clinical aspects, all these findings suggest a new treatment perspective is necessary.
We can conclude that CDK4/6 inhibitor therapy combined with radiotherapy is safe and the authors recommend this therapy for better and more effective outcomes appropriate sequence, which is not written in the official summary of product characteristics. Radiotherapy should be the initial treatment, followed by CDK4/6 inhibitors in de novo discovered cases; otherwise, a short suspension of the drug is advised to eliminate the CDK4/6 inhibitor from plasma and let the radiotherapy destroy cancerous cells arriving in the G2/M phase.
The adequate local ablative treatment in oligo-progression could be sufficient and, following this, we can maintain the otherwise effective systemic therapy. Therefore, we can extend and prolong the time interval of the metastatic first-line treatment with CDK4/6 inhibitor, even changing the endocrine partner (letrozole to fulvestrant). This is why it is important to ensure an effective local ablative treatment with radiotherapy in bone-only oligo-metastatic cases. If necessary, bone modifying agent can be shifted (bisphosphonate to denosumab) in another step. In the case of infectivity of these treatment changes, the main component of the first-line treatment (CDK4/6 inhibitor) should be relied on in the second-line treatment. With this soft change, patients can gain time and a good quality of life as we know that further line treatments are usually associated with more adverse events and lead to shorter progression-free intervals. In our daily practice, we deliver SBRT (stereotactic body radiation therapy) for two main reasons: stereotactic radiotherapy doses are potentially more lethal to metastases and may dominate the disease; meanwhile, SBRT requires a shorter treatment time.
Further questions can be addressed by scientists, such as “What about particle radiotherapy combined with targeted agents?” Preliminary data suggest that high LET radiation, such as heavy ion radiation like C ion irradiation may be a favorable partner to CDK4/6 inhibitor application [49]. Probably, this is the future hope when high-energy heavy ion radiation would be the essential part of cancer treatment and wide spread would be available [50].
All oncological treatment must be discussed in a multidisciplinary team and individualized options must be calculated, taking into account further possibilities and as far as possible, maintaining an effective, highly tolerable therapy for as long as we can with the hope of prolonging the good quality of post-limited-progression life.
Statements
Author contributions
Conceptualization, IT, PA, and AF; methodology, AF and PA; data curation, IT and AF; writing—original draft preparation, AF and IT; writing—review and editing, AF, IT, IH, and PA; visualization, IT; supervision, AF, PA, and IH. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca: Cancer J Clin (2021) 71(3):209–49. 10.3322/caac.21660
2.
Howlader N Altekruse SF Li CI Chen VW Clarke CA Ries LA et al US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. Jnci: J Natl Cancer Inst (2014) 106(5):dju055–8. 10.1093/jnci/dju055
3.
Bosacki C Bouleftour W Sotton S Vallard A Daguenet E Ouaz H et al CDK 4/6 inhibitors combined with radiotherapy: a review of literature. Clin Transl Radiat Oncol (2021) 26:79–85. 10.1016/j.ctro.2020.11.010
4.
Feng Y Spezia M Huang S Yuan C Zeng Z Zhang L et al Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis (2018) 5:77–106. 10.1016/j.gendis.2018.05.001
5.
Wu Q Li J Zhu S Wu J Chen C Liu Q et al Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget (2017) 8:27990–6. 10.18632/oncotarget.15856
6.
Svensson E Christiansen CF Ulrichsen SP RørthSørensen MRHT Sørensen HT . Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ open (2017) 7:e016022–7. 10.1136/bmjopen-2017-016022
7.
Cristofanilli M Turner NC Bondarenko I Ro J Im SA Masuda N et al Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol (2016) 17:425–39. 10.1016/S1470-2045(15)00613-0
8.
Tripathy D Im SA Colleoni M Franke F Bardia A Harbeck N et al Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol (2018) 19:904–15. 10.1016/S1470-2045(18)30292-4
9.
Johnston S Martin M Di Leo A Im SA Awada A Forrester T et al MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer (2019) 5:5–8. 10.1038/s41523-018-0097-z
10.
Johnston SR Harbeck N Hegg R Toi M Martin M Shao ZM et al Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol (2020) 38:3987–98. 10.1200/JCO.20.02514
11.
Xu B Zhang Q Zhang P Hu X Li W Tong Z et al Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat Med (2021) 27:1904–9. 10.1038/s41591-021-01562-9
12.
Yin L Yao Z Wang Y Huang YH Mazuranic M Yin A . 4MO Preclinical evaluation of novel CDK4/6 inhibitor GLR2007 in breast and lung cancer models. Ann Oncol (2021) 32:S362–362. 10.1016/j.annonc.2021.08.282
13.
Braal CL Jongbloed EM Wilting SM Mathijssen RH Koolen SL Jager A . Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs (2021) 81:317–31. 10.1007/s40265-020-01461-2
14.
Parkes A Clifton K Al-Awadhi A Oke O Warneke CL Litton JK et al Characterization of bone only metastasis patients with respect to tumor subtypes. NPJ Breast Cancer (2018) 4:2–7. 10.1038/s41523-018-0054-x
15.
Petroni G Galluzzi L . Impact of treatment schedule on the efficacy of cytostatic and immunostimulatory agents. Oncoimmunology (2021) 10:1889101–4. 10.1080/2162402X.2021.1889101
16.
Petroni G Buqué A Yamazaki T Bloy N Liberto MD Chen-Kiang S et al Radiotherapy delivered before CDK4/6 inhibitors mediates superior therapeutic effects in ER+ breast cancer. Clin Cancer Res (2021) 27:1855–63. 10.1158/1078-0432.CCR-20-3871
17.
Yang Y Luo J Chen X Yang Z Mei X Ma J et al CDK4/6 inhibitors: a novel strategy for tumor radiosensitization. J Exp Clin Cancer Res (2020) 39:188–11. 10.1186/s13046-020-01693-w
18.
Ades F Tryfonidis K Zardavas D . The past and future of breast cancer treatment—from the papyrus to individualised treatment approaches. Ecancermedicalscience (2017) 11:746–11. 10.3332/ecancer.2017.746
19.
Vozenin MC Baumann M Coppes RP Bourhis J . FLASH radiotherapy international workshop. Radiother Oncol (2019) 139:1–3. 10.1016/j.radonc.2019.07.020
20.
Yokoya A Shikazono N Fujii K Urushibara A Akamatsu K Watanabe R . DNA damage induced by the direct effect of radiation. Radiat Phys Chem (2008) 77:1280–5. 10.1016/j.radphyschem.2008.05.021
21.
O'neill P Fielden EM . Primary free radical processes in DNA. Adv Radiat Biol (1993) 17:53–120. 10.1016/B978-0-12-035417-7.50005-2
22.
Abraham RT . Checkpoint signalling: focusing on 53BP1. Nat Cel Biol (2002) 4:E277–9. 10.1038/ncb1202-e277
23.
Lin Y Raj J Li J Ha A Hossain MA Richardson C et al APE1 senses DNA single-strand breaks for repair and signaling. Nucleic Acids Res (2020) 48:1925–40. 10.1093/nar/gkz1175
24.
Galluzzi L Vitale I Aaronson SA Abrams JM Adam D Agostinis P et al Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ (2018) 25:486–541. 10.1038/s41418-017-0012-4
25.
Kumari S Mukherjee S Sinha D Abdisalaam S Krishnan S Asaithamby A . Immunomodulatory effects of radiotherapy. Int J Mol Sci (2020) 21:8151–29. 10.3390/ijms21218151
26.
Lim S Kaldis P . Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development (2013) 140:3079–93. 10.1242/dev.091744
27.
Pesch AM Hirsh NH Michmerhuizen AR Jungles KM Wilder-Romans K Chandler BC et al RB expression confers sensitivity to CDK4/6 inhibitor–mediated radiosensitization across breast cancer subtypes. JCI Insight (2022) 7:e154402–18. 10.1172/jci.insight.154402
28.
Filmus J Robles AI Shi W Wong MJ Colombo LL Conti CJ . Induction of cyclin D1 overexpression by activated ras. Oncogene (1994) 9:3627–33.
29.
Tanyi J Tory K Bánkfalvi A Shröder W Rath W Füzesi L . Analysis of p53 mutation and cyclin D1 expression in breast tumors. Pathol Oncol Res (1999) 5(2):90–4. 10.1053/paor.1999.0201
30.
Asghar U Witkiewicz AK Turner NC Knudsen ES . The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov (2015) 14:130–46. 10.1038/nrd4504
31.
Pesch AM Hirsh NH Chandler BC Michmerhuizen AR Ritter CL Androsiglio MP et al Short-term CDK4/6 inhibition radiosensitizes estrogen receptor-positive breast cancers. Clin Cancer Res (2020) 26:6568–80. 10.1158/1078-0432.CCR-20-2269
32.
Klapp V Buqué A Bloy N Sato A Yamazaki T Zhou XK et al Cellular senescence in the response of HR+ breast cancer to radiotherapy and CDK4/6 inhibitors. J Transl Med (2023) 21:110–0. 10.1186/s12967-023-03964-4
33.
Alomran R White M Bruce M Bressel M Roache S Karroum L et al Stereotactic radiotherapy for oligoprogressive ER-positive breast cancer (AVATAR). BMC Cancer (2021) 21:303–9. 10.1186/s12885-021-08042-w
34.
Meattini I Desideri I Scotti V Simontacchi G Livi L . Ribociclib plus letrozole and concomitant palliative radiotherapy for metastatic breast cancer. Breast (2018) 42:1–2. 10.1016/j.breast.2018.08.096
35.
David S Ho G Day D Harris M Tan J Goel S et al Enhanced toxicity with CDK 4/6 inhibitors and palliative radiotherapy: non-consecutive case series and review of the literature. Transl Oncol (2021) 14:100939–7. 10.1016/j.tranon.2020.100939
36.
Kubeczko M Gabryś D Gawkowska M Polakiewicz-Gilowska A Cortez AJ Krzywon A et al Safety and feasibility of radiation therapy combined with CDK 4/6 inhibitors in the management of advanced breast cancer. Cancers (2023) 15:690–17. 10.3390/cancers15030690
37.
Harbeck N Franke F Villanueva-Vazquez R Lu YS Tripathy D Chow L et al Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: results from a phase III randomized clinical trial (MONALEESA-7). Ther Adv Med Oncol (2020) 12:1758835920943065–8. 10.1177/1758835920943065
38.
Dickler MN Tolaney SM Rugo HS Cortés J Diéras V Patt D et al MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory hr+/HER2- metastatic breast cancer. Clin Cancer Res (2017) 23:5218–24. 10.1158/1078-0432.CCR-17-0754
39.
Sledge GW Jr Toi M Neven P Sohn J Inoue K Pivot X et al Monarch 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol (2017) 35:2875–84. 10.1200/JCO.2017.73.7585
40.
Goetz MP Toi M Campone M Sohn J Paluch-Shimon S Huober J et al Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol (2017) 35:3638–46. 10.1200/JCO.2017.75.6155
41.
Finn RS Crown JP Lang I Boer K Bondarenko IM Kulyk SO et al The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol (2015) 16:25–35. 10.1016/S1470-2045(14)71159-3
42.
Finn RS Martin M Rugo HS Jones S Im SA Gelmon K et al Palbociclib and letrozole in advanced breast cancer. N Engl J Med (2016) 375:1925–36. 10.1056/NEJMoa1607303
43.
Lelliott EJ Kong IY Zethoven M Ramsbottom KM Martelotto LG Meyran D et al CDK4/6 inhibition promotes antitumor immunity through the induction of T-cell memory. Cancer Discov (2021) 11:2582–601. 10.1158/2159-8290.CD-20-1554
44.
Galluzzi L Vitale I Warren S Adjemian S Agostinis P Martinez AB et al Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer (2020) 8:e000337–21. 10.1136/jitc-2019-000337
45.
Göttgens EL Bussink J Leszczynska KB Peters H Span PN Hammond EM . Inhibition of CDK4/CDK6 enhances radiosensitivity of HPV negative head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys (2019) 105:548–58. 10.1016/j.ijrobp.2019.06.2531
46.
Xie X Zheng W Chen T Lin W Liao Z Liu J et al CDK4/6 inhibitor palbociclib amplifies the radiosensitivity to nasopharyngeal carcinoma cells via mediating apoptosis and suppressing DNA damage repair. Onco Targets Ther (2019) 12:11107–17. 10.2147/OTT.S234221
47.
Al-Rashdan A Quirk S Roumeliotis M Abedin T Amaro CP Barbera L et al Radiation therapy with cyclin-dependent kinase 4/6 inhibitors: a multi-institutional safety and toxicity study. Int J Radiat Oncol Biol Phys (2022) 114:399–408. 10.1016/j.ijrobp.2022.07.005
48.
Rubovszky G Kocsis J Boér K Chilingirova N Dank M Kahán Z et al Systemic treatment of breast cancer. 1st central-eastern European professional consensus statement on breast cancer. Pathol Oncol Res (2022) 28:1–26. 10.3389/pore.2022.1610383
49.
Helm A Fournier C Durante M . Particle radiotherapy and molecular therapies: mechanisms and strategies towards clinical applications. Expert Rev Mol Med (2022) 24:e8–11. 10.1017/erm.2022.2
50.
Nikitaki Z Velalopoulou A Zanni V Tremi I Havaki S Kokkoris M et al Key biological mechanisms involved in high-LET radiation therapies with a focus on DNA damage and repair. Expert Rev Mol Med (2022) 24:e15–15. 10.1017/erm.2022.6
Summary
Keywords
breast cancer, radiotherapy, radiosensitivity, CDK4/6 inhibitors, treatment timing
Citation
Tornyi I, Árkosy P, Horváth I and Furka A (2023) A new perspective on the proper timing of radiotherapy during CDK4/6 inhibitor therapy in patients with “bone-only” metastatic breast cancer. Pathol. Oncol. Res. 29:1611369. doi: 10.3389/pore.2023.1611369
Received
23 June 2023
Accepted
25 September 2023
Published
11 October 2023
Volume
29 - 2023
Edited by
József Tímár, Semmelweis University, Hungary
Updates
Copyright
© 2023 Tornyi, Árkosy, Horváth and Furka.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ildikó Horváth, horvath.ildiko@med.unideb.hu, ildiko.horvath@koranyi.hu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.