Abstract
The renal angiomyolipoma (AML) is a benign tumor characteristically composed of fat, smooth muscle tissue, and vessels. We collected AMLs from our nephrectomy database, reclassified them according to their histological appearance, recorded the demographic, clinical, and pathological parameters, and compared them with oncocytoma (RO) and renal cell carcinoma (RCC). Immunohistochemistry was ordered in 41 cases. In 2224 nephrectomies, we found 52 AMLs with a 53 mm median size. The mean age was 52.76. Forty-eight tumors were sporadic, while four were hereditary. The revision resulted in 31 classic, 13 leiomyoma-like, five lipoma-like, two epithelioid, and one AML with epithelial cysts. SMA was diffusely positive, except for the epithelioid type, while MelanA harbored stronger expression than HMB45. AML was more frequent in females and appeared ten and 7 years earlier than RO and RCC, respectively. The follow-up time was 7.42 years, and neither tumor-related death nor relapse occurred. AML is rare in nephrectomies and develops primarily in females in their 50s with an average size of 50–60 mm at the surgery. The histological appearance in order of frequency is classic, leiomyoma-like, lipoma-like, epithelioid, and cystic. The MelanA, HMB45, and SMA immunohistochemistry can support the light-microscopic findings.
Introduction
The angiomyolipoma (AML) is a benign tumor occurring mainly in the kidney and belongs to the perivascular epithelioid cell tumors (PEComa) [1, 2]. Generally, AML is composed of thick-walled vessels, adipose, and smooth muscle tissue in various distributions [3], but occasionally, the tumor has cystic, leiomyoma-like, lipoma-like, or epithelioid appearance [4]. In contrast to classic AML, the latter has malignant potential, and a certain number of these cases may relapse or give distant metastasis [4]. AML can be sporadic or hereditary and linked to tuberous sclerosis (TSC) [5]. Sporadic tumors are four times more frequent in females, related to hormonal causes [6, 7]. Hereditary cases develop in younger individuals and have no gender predilection [4, 8]. Genetically speaking, AMLs are characterized by the biallelic inactivation of TSC1 or TSC2 genes [5, 9], which encode the hamartin and tuberin, respectively [5, 10]. These proteins, along with TBC1D7, build up the TSC1-TSC2 complex that regulates the cellular metabolism, protein synthesis, and cell cycle via the mTOR pathway [11]. AML occurs approximately in 1% of the nephrectomy specimens [4]. Most of the sporadic cases are discovered accidentally, but infrequently (2.2%), they may cause hemorrhage, which positively correlates with the tumor size [12, 13]. In a typical clinical scenario, the imaging technics can differentiate AMLs from renal cell tumors [14, 15], and for these cases, the radiologic follow-up might be adequate and beneficial. However, the usual sensitivity of radiological techniques is inappropriate in epithelioid and leiomyoma-like morphology [16]. Surgical removal is needed for tumor-related symptoms, compression of the adjacent structures, and hemorrhagic complications [17]. If applicable, nephron-sparing techniques should be used [17]. For classic AML, the pathological diagnosis is usually straightforward [4], but tumors with epithelioid [18] and leiomyoma-like [4] morphology often require extensive immunohistochemical examinations to exclude renal cell carcinoma (RCC), metastasis, and sarcoma. Multifocality and bilaterality are worrisome features for TSC; consequently, in the cases, clinical genetic consultation along with the investigation of the TSC1 and TSC2 are necessary [19]. Peripheral blood and buccal smear can be the source for the germline testing of the genes mentioned above [19]. We collected 52 consecutively removed AMLs and analyzed the patients’ clinical characteristics and the tumors’ pathological features.
Materials and methods
Case selection and pathological revision
AML cases were collected from the archive of the Department of Pathology, Albert Szent-Györgyi Medical School, University of Szeged. Two pathologists (AJ and LK) reviewed all hematoxylin eosin-stained slides and available immunohistochemical staining. They reclassified the cases according to the current classification scheme, and the subtypes were as follows: classic AML, AML with epithelial cysts (AMLEC), lipoma-like AML, leiomyoma-like AML, oncocytic AML, and epithelioid AML (eAML) [4]. In this study, solely nephrectomy samples were enrolled; therefore, biopsy and autopsy cases were excluded. The demographic data (age and gender) along with the main clinical features (symptoms and syndromic background) were collected. The tumors’ size, laterality, and multifocality were registered based on the original pathology report. These characteristics were compared with renal cell carcinoma (RCC) and oncocytoma (RO).
Immunohistochemistry
All immunohistochemical stains available in 22 tumors were reviewed. By using tissue microarray technique, another 19 AMLs were stained by MelanA (Labvision, clone A103, mouse monoclonal antibody, dilution 1:200), HMB45 (Cell Marque, clone hmb-45, mouse monoclonal antibody, dilution: 1:200), and SMA (Cell Marque, clone 1a4, mouse monoclonal antibody, dilution: 1:300). Two 2-mm-thick tissue cores represented the tumors. The reactions were evaluated in a semiquantitative fashion (0% positivity = negative; 1%–50% positivity = +; 51%–100% positivity = ++). The FFPE blocks were unavailable in eleven cases; hence, no immunohistochemistry was performed for these tumors.
Statistical analysis
For parametric and non-parametric tests, the SPSS software package was applied, and the differences were deemed significant if p < .05.
Results
Clinical aspects
Fifty-two AML cases were diagnosed from 2224 nephrectomy specimens. The mean age of all patients was 52.76 years (range 27–76 years). In males, 7 tumors, while, in females, 45 AMLs occurred (female-to-male ratio: 6.42:1). Forty-eight tumors were sporadic in our data set, and four were linked to TSC. The mean ages of sporadic and TSC cases were 54.04 and 37.75, respectively. One tumor developed in polycystic kidney disease, and another one evolved in a graft kidney. Tumor rupture and hemorrhagic complications occurred in eight patients (shown in Figure 1), and in one case, a hemorrhagic shock was developed as well. Fourteen tumors were resected, while total and radical nephrectomy was carried out in 30 and 8 patients, respectively. The median follow-up time was 2.64 years, and neither local relapse nor tumor-related death was registered. The patients’ clinical characteristics are summarized in Table 1.
FIGURE 1
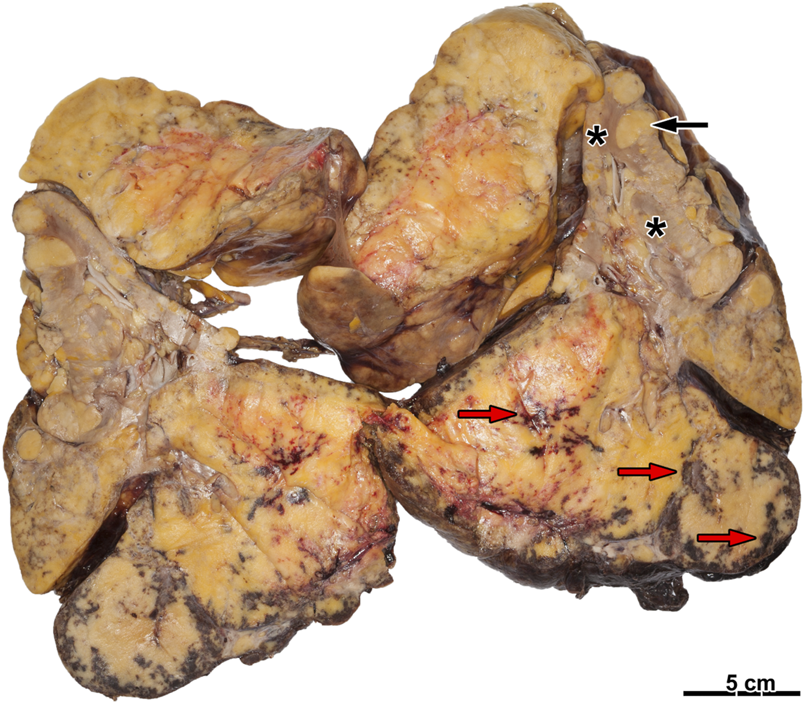
Ruptured angiomyolipoma with hemorrhage. There is a fatty tumor on the cut surface with several foci of hemorrhage (red arrow). On the other hand, several smaller tumor nodules are present (black arrow). The renal parenchyma is hard to recognize (asterisks).
TABLE 1
| Case | Age | Sex | Signs/Circumstances of discovery | Tuberous sclerosis | Surgery | Additional data |
|---|---|---|---|---|---|---|
| 1 | 52 | F | No data | No | Radical nephrectomy | - |
| 2 | 66 | F | Incidental finding at cholecystectomy | No | Tumor resection | - |
| 3 | 73 | F | Tumor rupture | No | Total nephrectomy | - |
| 4 | 36 | F | No data | No | Total nephrectomy | - |
| 5 | 46 | F | Tumor rupture | No | Total nephrectomy | - |
| 6 | 55 | F | Tumor rupture | No | Total nephrectomy | - |
| 7 | 58 | F | Retroperitoneal hemorrhage | No | Total nephrectomy | - |
| 8 | 33 | F | No data | Yes | Total nephrectomy | - |
| 9 | 26 | F | No data | No | Total nephrectomy | - |
| 10 | 49 | F | No data | No | Tumor resection | - |
| 11 | 26 | F | Surgical finding at kidney transplantation | No | Total nephrectomy | - |
| 12 | 45 | F | Incidental finding at SLE examination | No | Tumor resection | - |
| 13 | 34 | F | No data | Yes | Radical nephrectomy | - |
| 14 | 46 | F | No data | No | Total nephrectomy | - |
| 15 | 53 | F | No data | No | Radical nephrectomy | - |
| 16 | 47 | F | No data | No | Tumor resection | - |
| 17 | 62 | F | No data | No | Total nephrectomy | - |
| 18 | 60 | F | No data | No | Total nephrectomy | - |
| 19 | 52 | F | Tumor rupture | No | Radical nephrectomy | - |
| 20 | 49 | F | Incidental finding on abdominal US | No | Total nephrectomy | - |
| 21 | 49 | F | At the examination of hemorrhoids | No | Radical nephrectomy | - |
| 22 | 60 | F | Tumor rupture | No | Total nephrectomy | - |
| 23 | 57 | F | No data | No | Radical nephrectomy | - |
| 24 | 60 | F | Hematuria | No | Total nephrectomy | - |
| 25 | 67 | F | No data | No | Total nephrectomy | - |
| 26 | 35 | F | No data | No | Total nephrectomy | - |
| 27 | 56 | M | At the examination of kidney stones | No | Tumor resection | - |
| 28 | 31 | F | At the examination of PCOS | No | Tumor resection | - |
| 29* | 40 | F | Hemorrhagic shock | Yes | Total nephrectomy | - |
| 30 | 58 | F | Tumor rupture | No | Total nephrectomy | Evolved in horseshoe kidney |
| 31 | 43 | M | At the examination for kidney transplantation | No | Radical nephrectomy | Evolved in polycystic kidney |
| 32 | 66 | F | No data | No | Total nephrectomy | - |
| 33 | 73 | M | At the examination of BPH | No | Tumor resection | - |
| 34 | 76 | F | Incidental finding on abdominal US | No | Tumor resection | - |
| 35 | 54 | M | At graft kidney’s follow-up | No | Tumor resection | Evolved in graft kidney |
| 36 | 65 | F | No data | No | Total nephrectomy | - |
| 37 | 68 | M | No data | No | Total nephrectomy | - |
| 38 | 68 | F | No data | No | Total nephrectomy | - |
| 39 | 46 | M | At the examination of urethral discharge | No | Total nephrectomy | - |
| 40 | 75 | F | No data | No | Total nephrectomy | Ipsilateral RO is present |
| 41 | 69 | F | No data | No | Tumor resection | - |
| 42 | 56 | F | No data | No | Tumor resection | - |
| 43 | 74 | F | No data | No | Total nephrectomy | Ipsilateral ccRCC is present |
| 44* | 43 | F | Renal pain, tumor rupture | Yes | Radical nephrectomy | - |
| 45 | 65 | F | No data | No | Tumor resection | - |
| 46 | 33 | F | No data | No | Tumor resection | - |
| 47 | 31 | M | No data | No | Total nephrectomy | - |
| 48 | 57 | F | No data | No | Total nephrectomy | - |
| 49 | 46 | F | Renal colic | No | Total nephrectomy | Ipsilateral HOCT and ccRCC are present |
| 50 | 60 | F | At gastrointestinal examination | No | Tumor resection | - |
| 51 | 54 | F | Subcostal pain | No | Total nephrectomy | - |
| 52 | 41 | F | Incidental finding on abdominal US | No | Tumor resection | - |
The clinical features of the cases investigated.
F, Female; M, Male; SLE, Systemic lupus erythematosus; US, Ultrasound; PCOS, Polycystic ovary syndrome; BPH, Benign prostatic hyperplasia; RO, Renal oncocytoma; ccRCC, Clear cell renal cell carcinoma; RCC-U, Renal cell carcinoma unclassified. * Case #29 and #44 are from the same patient.
Pathological aspects
The median size of all tumors was 53 mm (range: 4–300 mm), but in cases with tuberous sclerosis, it was found to be 260 mm. The revision of the cases resulted in thirty-one classic, thirteen leiomyoma-like, five lipoma-like, two epithelioid, and one AMLEC. No oncocytic AML was seen. Synchronous tumors were observed in four cases (Table 1). Seven AMLs were multifocal. A renal sinus invasion was present in case #37 and #51 along with the infiltration of the renal vein in the latter. Both cases harbored epithelioid morphology. Besides, microscopic tumor necrosis was solely present in eAMLs. At least one melanocytic and smooth muscle marker was expressed in every case. Diffuse SMA-positivity was seen in all tumors except the eAMLs. Among the melanocytic markers, the MelanA was stronger (+ = 16; ++ = 18) compared to HMB45 (+ = 27, ++ = 7). Ki67 immunohistochemistry was available for six tumors, and the mean proliferation activity was about 1%. The morphological aspects are summarized in Table 2.
TABLE 2
| Case | Laterality | Focality | Size (mm) | Histological subtype | Immunohistochemistry |
|---|---|---|---|---|---|
| 1 | Right | Unifocal | 20 | Leiomyoma-like | MelanA: ++, HMB45: +, SMA: ++ |
| 2 | Right | Unifocal | 6 | Leiomyoma-like | MelanA: ++, HMB45: +, SMA: ++ |
| 3 | Right | Unifocal | No data | Classic | MelanA: -, HMB45: +, SMA: ++ |
| 4 | Left | Unifocal | 40 | Classic | MelanA: +, HMB45: +, SMA: ++ |
| 5 | Left | Unifocal | 75 | Classic | MelanA: ++, HMB45: +, SMA: ++ |
| 6 | Right | Unifocal | 20 | Classic | MelanA: ++, HMB45: −, SMA: ++ |
| 7 | Right | Unifocal | 39 | Leiomyoma-like | MelanA: ++, HMB45: +, SMA: ++ |
| 8 | Left | Multifocal | No data | Classic | MelanA: ++, HMB45: ++, SMA: ++ |
| 9 | Right | Unifocal | 36 | Leiomyoma-like | MelanA: ++, HMB45: ++, SMA: ++ |
| 10 | Left | Unifocal | 70 | Lipoma-like | MelanA: +, HMB45: +, SMA: ++ |
| 11 | Left | Multifocal | No data | Classic | Not performed |
| 12 | Right | Unifocal | 22 | Classic | MelanA: +, HMB45: +, SMA: ++ |
| 13 | Left | Multifocal | 200 | Classic | MelanA: ++, HMB45: ++, SMA: ++ |
| 14 | Left | Multifocal | 25 | Classic | Not performed |
| 15 | Right | Unifocal | 90 | Classic | Not performed |
| 16 | Right | Unifocal | 20 | Classic | Not performed |
| 17 | Left | Unifocal | 17 | Leiomyoma-like | HMB45: +, SMA: ++ |
| 18 | Left | Unifocal | 25 | Classic | Not performed |
| 19 | Left | Unifocal | 46 | Classic | MelanA: ++, HMB45: ++, SMA: ++ |
| 20 | Right | Unifocal | 170 | Lipoma-like | Not performed |
| 21 | Left | Unifocal | 65 | Classic | Not performed |
| 22 | Left | Unifocal | 245 | Classic | Not performed |
| 23 | Left | Unifocal | 100 | Classic | MelanA: +, HMB45: +, SMA: ++ |
| 24 | Right | Unifocal | 30 | Leiomyoma-like | Not performed |
| 25 | Right | Unifocal | 40 | Classic | MelanA: +, HMB45: −, SMA: ++ |
| 26 | Left | Unifocal | 80 | Classic | MelanA: −, HMB45: +, SMA: ++ |
| 27 | Left | Unifocal | 37 | Leiomyoma-like | HMB45: +, SMA: ++ |
| 28 | Right | Unifocal | 40 | Classic | MelanA: ++, HMB45: ++, SMA: ++, CD1a: −, Ki67: 1% |
| 29 | Right | Multifocal | 300 | Classic | MelanA: +, HMB45: +, SMA: ++ |
| 30 | Right | Unifocal | 155 | Classic | MelanA: +, HMB45: +, SMA: ++ |
| 31 | Left | Multifocal | 6 | Classic | MelanA: ++, HMB45: +, SMA: ++ |
| 32 | Left | Unifocal | 42 | Leiomyoma-like | MelanA: +, HMB45: +, SMA: ++, h-Caldesmon: ++ |
| 33 | Left | Unifocal | 20 | Classic | MelanA: +, HMB45: +, SMA: ++, CathepsinK: + |
| 34 | Left | Unifocal | 31 | Leiomyoma-like | MelanA: ++, HMB45: ++, SMA: ++ CD1a: − |
| 35 | Right | Unifocal | 45 | Lipoma-like | MelanA: ++, HMB45: ++, SMA: ++ |
| 36 | Right | Unifocal | No data | Classic | Not performed |
| 37 | Left | Unifocal | 40 | Epitheloid | MelanA: +, HMB45: +, SMA: +, EMA: −, PAX2: −, S100: −, CD56: −, Ki67: 1% |
| 38 | Left | Unifocal | 69 | Classic | MelanA: ++, HMB45: -, SMA: ++ |
| 39 | Right | Unifocal | 20 | Cystic | Epithel: CK7: ++, PAX8: ++, MNF116: ++, Stroma: MelanA: +, HMB45: +, SMA: ++, ER: +, PR: +, TLE1: −, S100: −, CD34: −, Ki67: <1% |
| 40 | Right | Unifocal | 4 | Classic | MelanA: +, HMB45: +, SMA: ++ |
| 41 | Left | Unifocal | 23 | Leiomyoma-like | MelanA: +, HMB45: −, SMA: ++ |
| 42 | Left | Unifocal | 46 | Classic | MelanA: ++, HMB45: −, SOX10: -, SMA: ++ |
| 43 | Right | Unifocal | 11 | Leiomyoma-like | MelanA: +, HMB45: −, SMA: ++ |
| 44 | Left | Unifocal | 260 | Classic | MelanA: ++, HMB45: +, SMA: ++ |
| 45 | Right | Unifocal | 30 | Lipoma-like | Not performed |
| 46 | Graft | Unifocal | 5 | Lipoma-like | MelanA: +, HMB45: +, SMA: ++, S100: ++, CD34: -, Ki67: <1% |
| 47 | Left | Unifocal | 52 | Leiomyoma-like | HMB45: +, SMA: ++, CD117: +, Ki67: 1%–2% |
| 48 | Right | Unifocal | 28 | Classic | HMB45: +, SMA: ++ |
| 49 | Left | Multifocal | 4 | Classic | MelanA: −, HMB45: ++, PAX8: −, SDHB: ++, β-Catenin: ++ (membrane), SMA: ++ |
| 50 | Left | Unifocal | 15 | Leiomyoma-like | MelanA: ++, HMB45: +, SMA: ++, Ki67: 1%–2% |
| MelanA: +, HMB45: -, SMA: +, CD68: +, CathepsinK: +, CD117: +, PAX2: − | |||||
| 51 | Left | Unifocal | 42 | Epitheloid | PAX8: −, FH: ++, SDHB: ++, CA9: −, CK7: −, CD10: −, TFE3: −, GATA3: − |
| PBRM1: ++, BAP1: ++ | |||||
| 52 | Left | Unifocal | 55 | Classic | MelanA: ++, HMB45: +, SMA: ++ |
The pathological characteristics of the cases investigated.
− = negative (0% positivity), + = 1%–50% positivity, ++ = 51%–100% positivity.
HMB45, Human melanoma black 45; SMA, Smooth muscle actin; CD, Cluster of differentiation; EMA, Epithelial membrane antigen; PAX, Paired-box; ER, Estrogen receptor; PR, Progesterone receptor; TLE1, Transducin-like enhancer of split 1; SOX10, SRY-related HMG-box 10; SDHB, Succinate dehydrogenase B subunit; FH, Fumarate hydratase; CA9, Carbonic anhydrase 9; CK7, Cytokeratin 7; TFE3, Transcription factor 3E; GATA3, GATA-binding factor 3; PBRM1, Polybromo 1; BAP1, BRCA1 associated protein 1.
Classic AML
Classic AML was the most common subtype (59.61%). Two tumors occurred in males (case #31 and #33). In case #35, the tumor developed in damaged kidney parenchyma (polycystic kidney), while, in case #33, the patient used finasteride to treat benign prostatic hyperplasia. All TSC-linked cases showed classic morphology. Figure 2 presents the morphological features of this subtype. Here, the median size of the tumors was 52 mm (range: 4–300 mm).
FIGURE 2
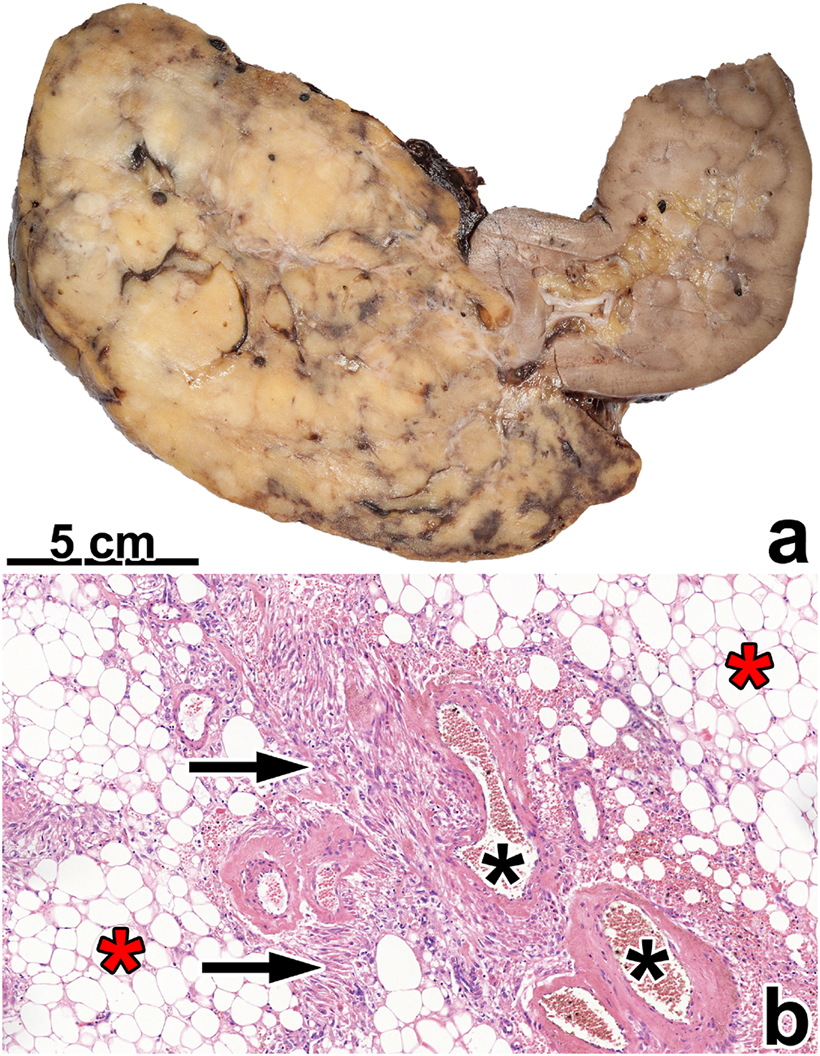
Classic angiomyolipoma. (A) The tumor is separated from the kidney parenchyma and has a fat tissue-like appearance. (B) Histologically, the tumor contains fat tissue (red asterisks), smooth muscle cells (black arrow), and thick blood vessels with hyaline walls (black asterisks). The image has a magnification factor of ×100.
Leiomyoma-like AML
This morphology was seen in 25% of the AMLs investigated. These tumors contained only a small amount of fat tissue; therefore, macroscopically, they caused no impression of AML (shown in Figure 3).
FIGURE 3
Leiomyoma-like angiomyolipoma. (A) Here, a greyish and whitish mass is present in the upper pole of the kidney, which is separated from the renal parenchyma and penetrates expansively to the adipose capsule. (B) The tumor is built up of spindle-shaped cells and blood vessels with thick walls (black arrow). The image has a magnification factor of ×100. (C,D) The SMA and MelanA immunostainings are diffusely positive. The two images have a magnification factor of ×200.
Lipoma-like AML
We registered a lipoma-like appearance in five cases, and case #35 evolved in a transplanted kidney. Figure 4 summarizes the morphological characteristics of this subtype.
FIGURE 4
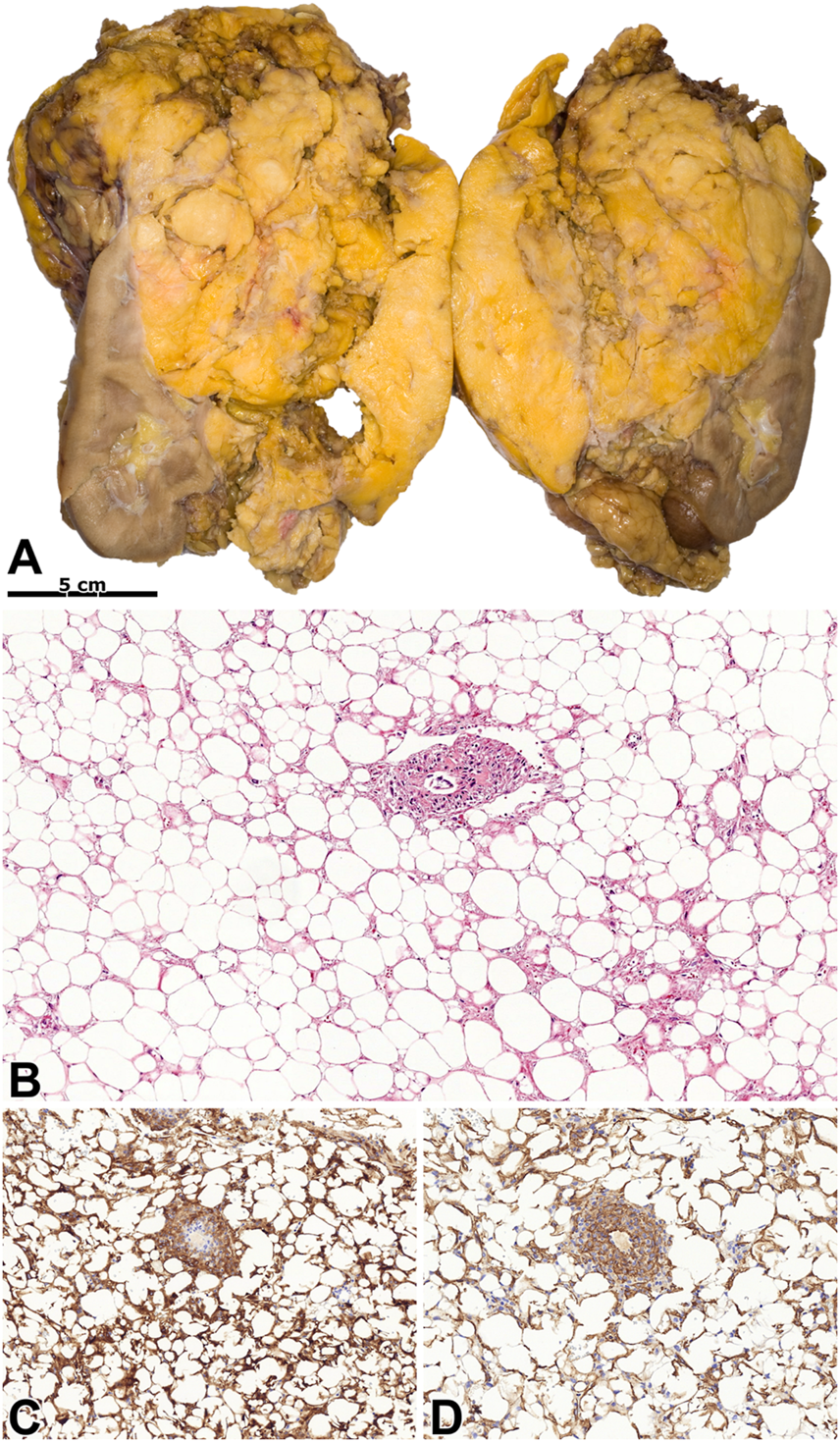
Lipoma-like angiomyolipoma. (A) An extensive mass with a fat-like appearance can be seen on the kidney’s cut surface. (B) The tumor histologically resembles lipoma, and blood vessels are occasionally seen. Besides, there are foci of smooth muscle cells among the adipocytes. The image has a magnification factor of ×100. (C,D) The tumor cells have diffuse co-expression (insert) of SMA and MelanA. The two images have a magnification factor of ×200.
Epithelioid AML
We diagnosed two tumors as eAML. This subtype required several immunohistochemical staining at the original histological diagnosis. Invasion, mitotic activity, and tumor cell necrosis were exclusively seen in these tumors (shown in Figure 5).
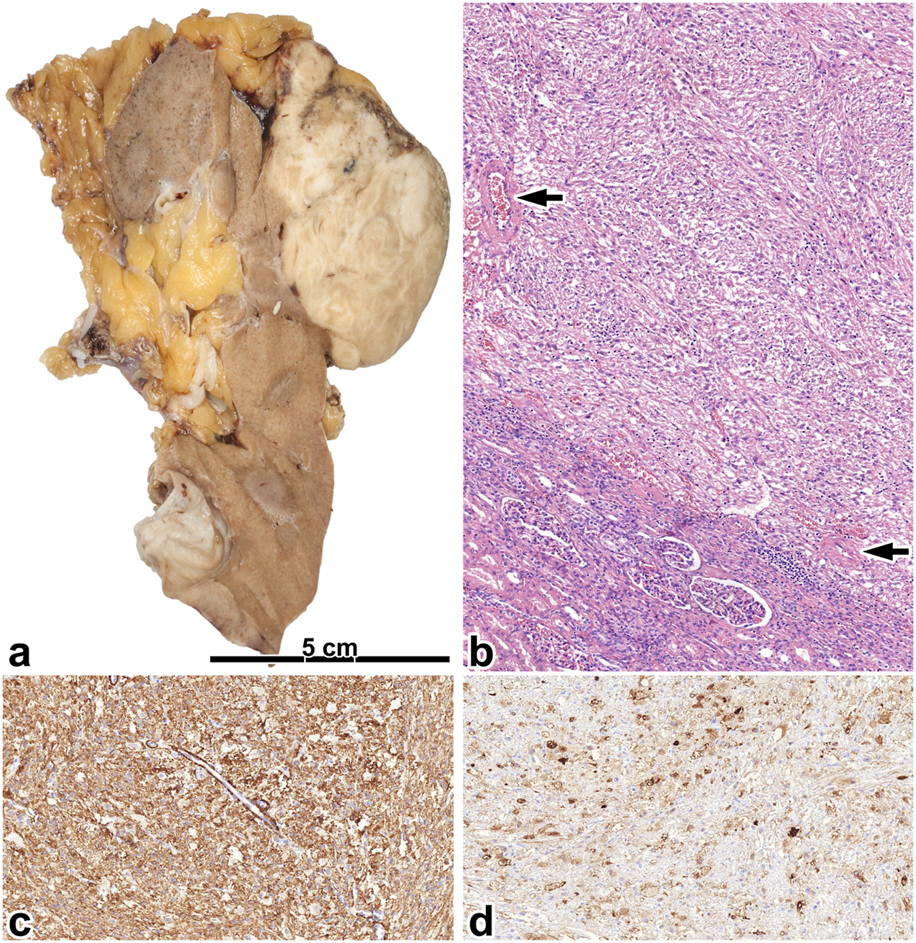
FIGURE 5
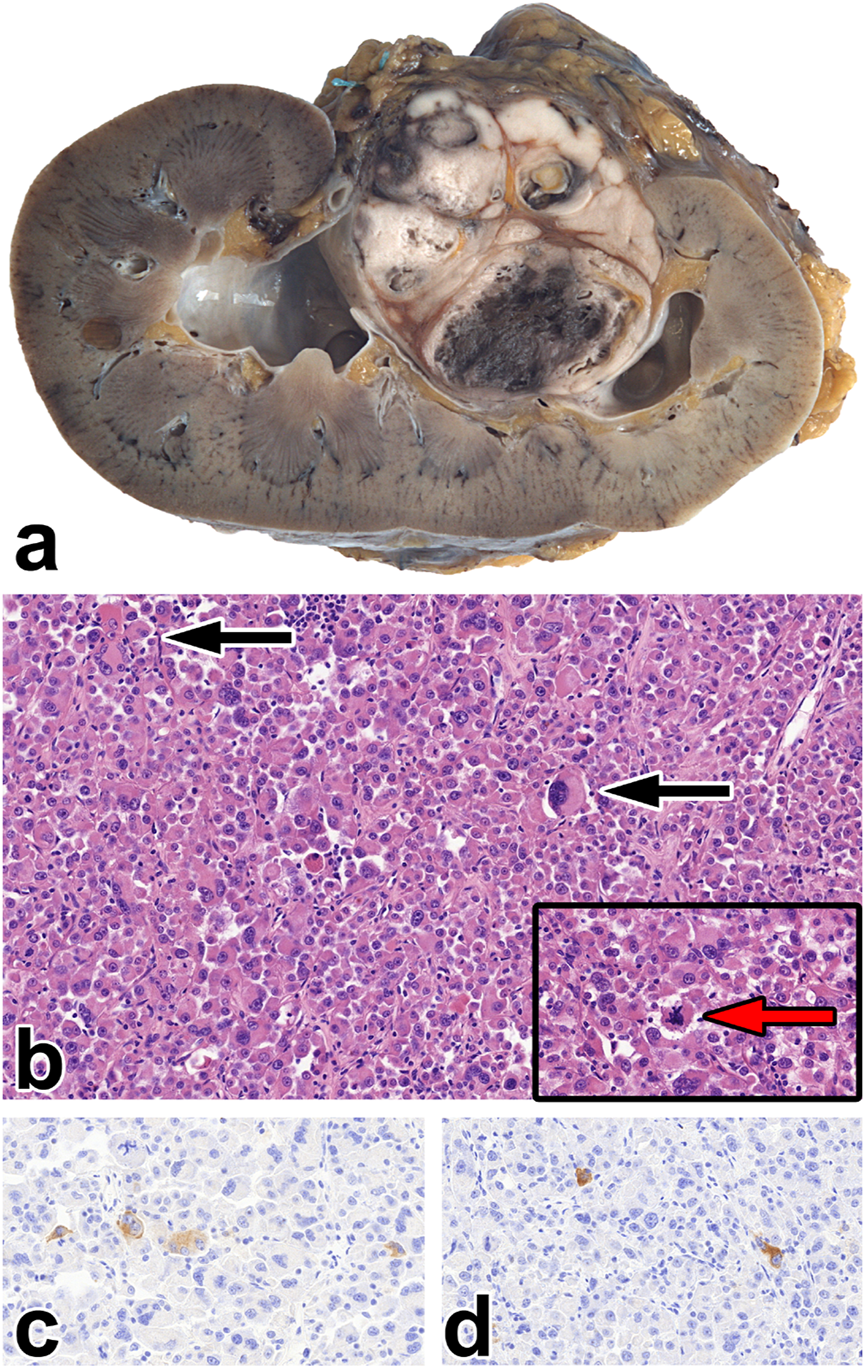
Epithelioid angiomyolipoma. (A) The gross picture shows a necrotic, hemorrhagic tumor located deeply in the renal sinus. (B) The tumor is made up of rhabdomyoblast-like cells. Besides, giant cells can be seen (black arrow), along with atypical mitosis (insert red arrow). The images have a magnification factor of ×200 and ×600, respectively. (C,D) MelanA and SMA co-expression is observed in some tumor cells. The two images have a magnification factor of ×400.
AML with epithelial cysts
This tumor was discovered in a 46-years-old male. The relatively small lesion was accidentally noticed during a urological investigation. Histologically, the tumor shared some characteristics with metanephric stromal tumor, and the tumor cells were estrogen and progesterone receptor-positive. We have no data on any hormonal treatment. Figure 6 represents the histological features of AMLEC.
FIGURE 6
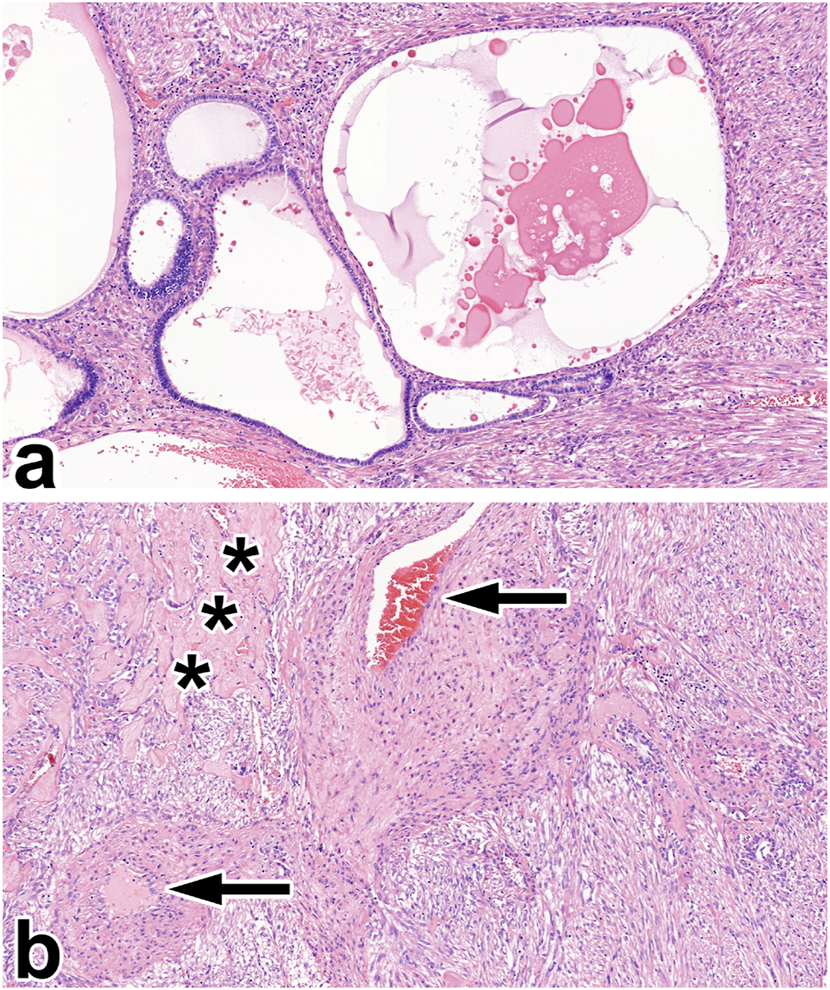
Angiomyolipoma with epithelial cysts. (A) The two-component tumor is built up of varying cysts in size and smooth muscle-rich stroma. (B) Apart from the smooth muscle cells, the stroma contains thick-walled blood vessels (black arrow) and sclerotic foci (black asterisks). All images have a magnification factor of ×100.
Correlation with different renal neoplasms
We compared the gender, age and tumor size of AML patients with those, who were operated with oncocytoma (RO) and renal cell carcinoma (RCC). The AML is more common in females (AML vs. RO; p < .001; AML vs. RCC p < .001). Concerning the age among AML, onocytoma and RCC patients, the AML occurs 10 years and 7 years prior to the two aforementioned tumors, respectively (AML [mean age = 52.76] vs. RO [mean age = 62.41], p < .001; AML [mean age = 52.76] vs. RCC [mean age = 59.58], p < .001). There is no difference in terms of the tumor size among the three tumors (AML [mean size = 59.56 mm] vs. RO [mean size = 48.3 mm], p = .78; AML [mean size = 59.56 mm] vs. RCC [mean size = 62.61 mm], p = .571).
Discussion
Oncocytoma and AML are the most common benign tumors of the kidney [4]. The former originates from the collecting ducts, while the latter comes from the perivascular epithelioid cells; hence it belongs to the PEComa tumor group [4]. PEComas include lymphangioleiomyomatosis, lung clear cell (sugar) tumor, and abdominopelvic PEComa [20, 21]. These tumors have a myomelanocytic differentiation with an expression of smooth muscle and melanocytic markers [1,2,3,4]. Based on the literature data, approximately 1% of nephrectomies are carried out due to AML [14]. We had a slightly higher incidence rate (2.33%). The difference might have two causes. At first, the specimens studied are collected for a relatively long period of time (1978–2021), and secondly, during nearly 45 years, the availability and sensitivity of the imaging techniques evolved significantly. The AML can be sporadic or linked to TSC [4]. The former is more frequent in females (female-to-male ratio = approximately 4:1) [22, 23]. This difference might have hormonal causes since the tumor cells in AML can express hormone receptors; moreover, in pregnant, the underlying AMLs can start a rapid enlargement [6, 7, 24]. We also found female dominance in our material with a female-to-male ratio of 6.14:1. Some authors suggest the syndromic cases lack the gender difference [12], while others state the opposite [4]. In our case set, all AMLs linked to TSC involved females. AML can occur at any age, but sporadic cases are mostly diagnosed at the end of the fourth or in the beginning of the fifth decades [6, 8]. We had a similar observation: our sporadic cases’ mean age was 52.76, and on the other hand, TSC-linked AMLs developed 15 years earlier (mean age: 37.75). Also, we found that the sporadic AMLs appear approximately 10 years earlier than RCC; therefore, in unsure renal tumor cases, for female patients in this age group, a renal biopsy can be advised to achieve the best patient care. For childhood AML cases, genetic consultation and studies are required [8]. AMLs usually have no symptoms, and they are incidental findings of examinations for other underlying disorders (i.e., hypertension, staging of malignant tumor) [4, 25]. According to the literature data, tumors larger than 40 mm induce symptoms in 80% of the cases. The common symptoms are groin pain, hematuria, and newly recognized high blood pressure [26]. Hemorrhagic complications are detected more frequently in tumors larger than 40 mm [12, 25, 27]. Limited anamnestic data were available in our study. A hemorrhagic complication was reported in eight patients, one of whom developed a life-threatening hemorrhagic shock. The average size of such tumors in our material was 110 mm. Most AMLs are radiologically safe to diagnose. Like the pathological classification, imaging diagnostics classify AML into several groups, including fat tissue-rich AML, fat tissue-poor AML, and fat tissue-invisible [28]. The fat tissue-rich AML is the largest group, corresponding to the classical type known from the pathological classification. By B-mode ultrasound, fat tissue-rich AML is described as a typical, homogeneous, hyperechoic lesion with no signs of necrosis or calcification [28]. The non-enhanced CT scan also gives a typical picture (shown in Figure 7) because one can measure fat density, i.e., values below -10 HU [29]. The MRI examination may help to distinguish between AML and RCC, as the loss of signal intensity between in-phase and out-of-phase sequences indicates the presence of microscopic fat tissue [30]. The tumor is usually separated from the kidney parenchyma, renal sinus, and adipose capsule but may appear in the renal vein or the regional lymph nodes [26, 31, 32]. The latter refers to a multicentric origin rather than metastasis [33]. The composition of the tumor influences the macroscopic appearance. The classic and lipoma-like AMLs have an adipose tissue-like cut surface, whereas leiomyoma-like AML and AMLEC mostly form a greyish-whitish mass [34]. The tumor is usually unilateral and unifocal, but 1/3 of the cases are multifocal, and 15% of the AMLs are bilateral [35]. Seven of our cases had multiple foci, and one patient with TSC had a bilateral tumor. In classic AML, the tumor consists of fat tissue, smooth muscle tissue, and thick-walled, irregular blood vessels in nearly similar proportions [36]. The adipose component corresponds to mature fat tissue, but sometimes vacuolated cells resembling lipoblasts can be detected. The smooth muscle cells typically form irregular fascicles and circular growth from the vessel wall [37]. The cytological atypia is usually mild, but sometimes, bizarre and multinucleated cells are present. The vascular component resembles thick-walled artery-like vessels with a thin elastic layer [4, 38]. The pathological diagnosis is straightforward in classic morphology, and immunostainings are unnecessary. Leiomyoma-like AMLs are often located below the fibrous capsule [39]. Because of the low-degree fat tissue component, the possibility of leiomyoma or schwannoma may arise; however, these, like other benign soft tissue tumors, are rare in the kidney and lack a myomelanocytic immunophenotype [40, 41]. In contrast to the former subtype, in the lipoma-like AML, the smooth muscle component may have a small amount, and the tumor is composed almost exclusively of fat tissue [42]. Such tumors are often associated with the adipose capsule and should be discriminated from lipoma or atypical lipomatous tumor [43]. These tumors are not characterized by myomelanocytic immunophenotype; furthermore, overexpression of MDM2 and CDK4 are seen in the latter [44]. The AMLEC is composed of stromal and epithelial components. The former is neoplastic, typically with irregular, smooth muscle bundles. The epithelial component forms cysts of varying size, but these most likely correspond to entrapped and dilated nephron segments [45, 46]. Currently, the term of angiomyolipoma with epithelial cysts is used instead of cystic AML [47]. This subtype is characterized by hormone receptor positivity, and accordingly, the tumor should be distinguished from the mixed epithelial and stromal tumor of the kidney (MESTK) [45]. The stroma of the two entities differs because, in MESTK, it mimics the ovarian stroma. Also, MESTK has no co-expression of melanocytic and smooth muscle markers [48]. From a clinical point of view, the identification of eAML is crucial, because in contrast to the subtypes discussed so far, this variant may recur in some cases and give metastases [49]. The incidence of malignant behavior is quite different in the literature, but the likelihood is approximately 5% based on two large-number studies [37, 49]. Some epithelioid component is present in all AMLs, but if its proportion is above 80%, the tumor must be diagnosed as eAML [49, 50]. The pure eAML is rare and can cause severe diagnostic difficulties as it should be distinguished mainly from rhabdoid RCC, translocation RCC, primary renal pleomorphic sarcoma, and metastasis; therefore, a large amount of immunohistochemistry is usually performed [51, 52]. Of note, the myomelanocytic phenotype can also be observed, but it is often present focally [49]. Genetically, all types of AML are characterized by the inactivation of the TSC1 or TSC2, which impairs the regulation of the mTOR signaling pathway and leads to increased cell proliferation [53]. A TP53 mutation has also been noted in eAML, possibly contributing to the malignant clinical course [54]. Genetic testing is only required if TSC is suspected. Immunomorphologically, AML is usually diffusely positive with SMA, and other muscle markers expression is seen in 50% of cases [49]. Expression of a melanocytic marker is also always observed, most commonly MelanA or HMB45. In a comparative study of 20 cases, all AMLs were diagnosed using these two markers, and other melanocytic markers (tyrosinase, CD117, NK1-C3, etc.) have little diagnostic benefit [55]. Focal SMA staining was present in our material only in eAML, and 75.6% of the study cases expressed both melanocytic markers, of which MelanA immunostaining was generally more extensive and more often positive. Immunohistochemistry of hamartin and tuberin has no diagnostic value [56]. The treatment is influenced by tumor size, bilaterality, and the possibility of malignancy. Asymptomatic tumors below 40 mm should be monitored by annual CT or MRI [57]. A closer observation is recommended in the range of 40–80 mm; usually, surgical treatment is performed in half of the patients due to complications [57]. Surgical removal should be advised in symptomatic cases, preferably using nephron-sparing techniques [58]. The tumors larger than 80 mm have a high risk of hemorrhagic complications and should be treated surgically or with radiologic intervention, such as selective arterial embolization or radiofrequency ablation [59]. The complications and stress are less severe in intervention, but these patients should be followed because tumors may recur [60]. To maintain renal function, a nephron-sparing resection is also preferred in bilateral cases [61]. In doubtful cases, a biopsy is required as a first step, and depending on the diagnosis, observation or surgery should be carried out [57, 62]. Systemic treatment is used for metastatic eAML, which may be chemotherapy, mTOR inhibitors, or immunotherapy [63, 64].
FIGURE 7
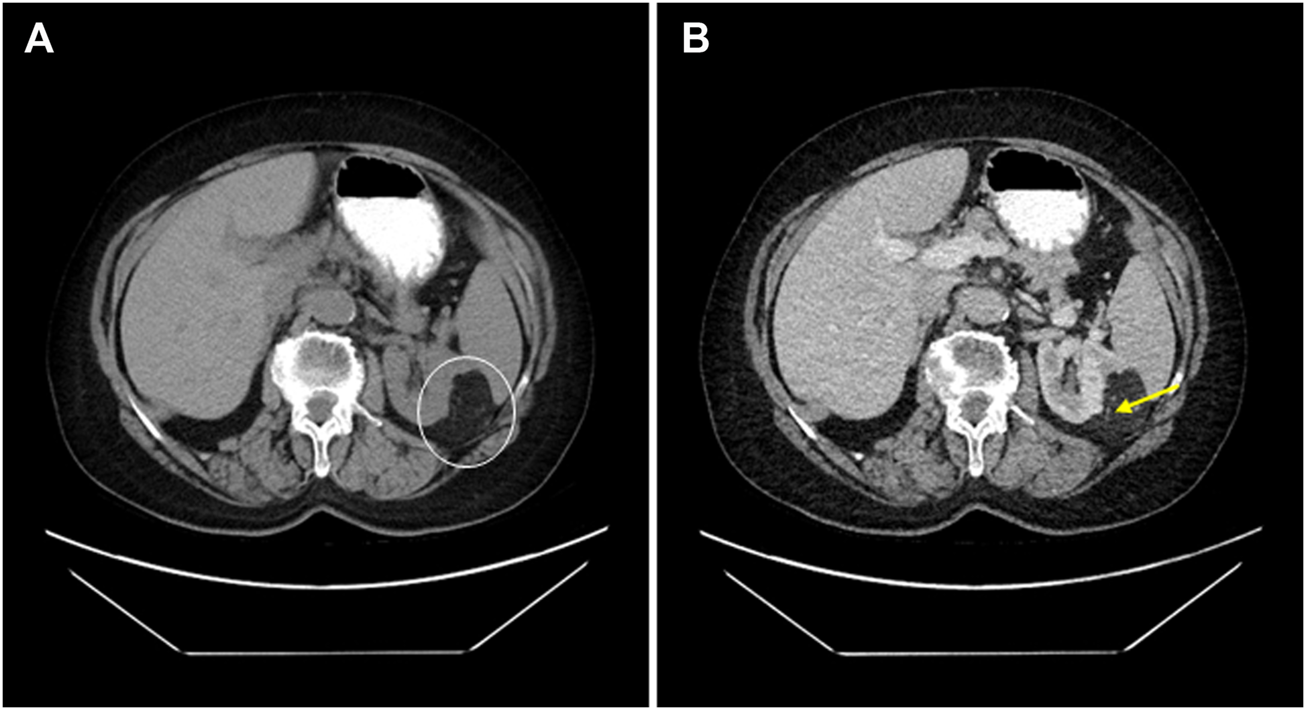
68-year-old female patient’s CT scan accidentally reveals an angiomyolipoma. (A) A native CT scan shows a lobulated 54 mm maximal axial diametric mass with a mean density of -79 HU (white circle). (B) In the same plane, the venous phasic cross-section shows that the difference does not change substantially; moreover, the thick-wall blood vessels become visible (yellow arrow).
Conclusion
AML is rare in nephrectomy specimens, and it is either sporadic or associated with TSC. It typically develops in women around 50 years, and the average size of surgically treated cases is about 50–60 mm. Histologically, the tumor may be classic, leiomyoma-like, lipoma-like, epithelioid, or cystic, in order of frequency. The eAML may be malignant, so such tumors should be treated like RCC and closely monitored. The pathological diagnosis is usually problem-free, and the light-microscopic findings may be supplemented by MelanA, HMB45, and SMA immunostaining.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Regional and Institutional Human Medical Biological Research Ethics Committee, University of Szeged. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZF prepared the figures and wrote the manuscript. IK and LV provided the patient characteristics and follow-up data. AJ and FS performed the histological analysis and immunohistochemical evaluation. LK contributed to the study concept and final supervision of the manuscript.
Funding
The University of Szeged, Faculty of Medicine Research Fund-Hetényi Géza Grant (Grant No. 5S 340 A202) and the New National Excellence Program funded this research (Grant No. UNKP-21-4-SZTE-131, UNKP-22-4-SZTE-305).
Acknowledgments
The authors gratefully acknowledge the assistance of Vivien Sánta and Samir Martin in the language editing of the text.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Folpe AL Kwiatkowski DJ . Perivascular epithelioid cell neoplasms: Pathology and pathogenesis. Hum Pathol (2010) 41(1):1–15. 10.1016/j.humpath.2009.05.011
2.
Martignoni G Pea M Reghellin D Zamboni G Bonetti F . PEComas: The past, the present and the future. Virchows Arch (2008) 452(2):119–32. 10.1007/s00428-007-0509-1
3.
Martignoni G Pea M Reghellin D Zamboni G Bonetti F . Perivascular epithelioid cell tumor (PEComa) in the genitourinary tract. Adv Anat Pathol (2007) 14(1):36–41. 10.1097/PAP.0b013e31802e0dc4
4.
WHO Classification of Tumours Editorial Board. Urinary and male genital tumours. In: WHO classification of tumours series. 5th ed.; vol. 8. Lyon (France): International Agency for Research on Cancer (2022).
5.
van Slegtenhorst M de Hoogt R Hermans C Nellist M Janssen B Verhoef S et al Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science (1997) 277(5327):805–8. 10.1126/science.277.5327.805
6.
Eble JN . Angiomyolipoma of kidney. Semin Diagn Pathol (1998) 15(1):21–40.
7.
Henske EP Ao X Short MP Greenberg R Neumann HP Kwiatkowski DJ et al Frequent progesterone receptor immunoreactivity in tuberous sclerosis-associated renal angiomyolipomas. Mod Pathol (1998) 11(7):665.
8.
Cook JA Oliver K Mueller RF Sampson J . A cross sectional study of renal involvement in tuberous sclerosis. J Med Genet (1996) 33(6):480–4. 10.1136/jmg.33.6.480
9.
Smolarek TA Wessner LL McCormack FX Mylet JC Menon AG Henske EP et al Evidence that lymphangiomyomatosis is caused by TSC2 mutations: Chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet (1998) 62(4):810–5. 10.1086/301804
10.
European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell (1993) 75(7):1305–15. 10.1016/0092-8674(93)90618-z
11.
Dibble CC Elis W Menon S Qin W Klekota J Asara JM et al TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cel (2012) 47(4):535–46. 10.1016/j.molcel.2012.06.009
12.
Steiner MS Goldman SM Fishman EK Marshall FF . The natural history of renal angiomyolipoma. J Urol (1993) 150(6):1782–6. 10.1016/s0022-5347(17)35895-0
13.
Yamakado K Tanaka N Nakagawa T Kobayashi S Yanagawa M Takeda K . Renal angiomyolipoma: Relationships between tumor size, aneurysm formation, and rupture. Radiology (2002) 225(1):78–82. 10.1148/radiol.2251011477
14.
Fujii Y Ajima J Oka K Tosaka A Takehara Y . Benign renal tumors detected among healthy adults by abdominal ultrasonography. Eur Urol (1995) 27:124–7. 10.1159/000475142
15.
Halpenny D Snow A McNeill G Torreggiani WC . The radiological diagnosis and treatment of renal angiomyolipomacurrent status. Clin Radiol (2010) 65(2):99–108. 10.1016/j.crad.2009.09.014
16.
Varma S Gupta S Talwar J Forte F Dhar M . Renal epithelioid angiomyolipoma: A malignant disease. J Nephrol (2011) 24(1):18–22. 10.5301/jn.2010.5451
17.
Kyo CK Won TK Won SH Jin SL Hee JJ Young DC . Trends of presentation and clinical outcome of treated renal angiomyolipoma. Yonsei Med J (2010) 51(5):728–34. 10.3349/ymj.2010.51.5.728
18.
Zavala-Pompa A Folpe AL Jimenez RE Lim SD Cohen C Eble JN et al Immunohistochemical study of microphthalmia transcription factor and tyrosinase in angiomyolipoma of the kidney, renal cell carcinoma, and renal and retroperitoneal sarcomas: Comparative evaluation with traditional diagnostic markers. Am J Surg Pathol (2001) 25(1):65–70. 10.1097/00000478-200101000-00007
19.
Amin S Kingswood JC Bolton PF Elmslie F Gale DP Harland C et al The UK guidelines for management and surveillance of Tuberous Sclerosis Complex. QJM (2019) 112(3):171–82. 10.1093/qjmed/hcy215
20.
Travis WD Brambilla E Nicholson AG Yatabe Y Austin JHM Beasley MB et al The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol (2015) 10(9):1243–60. 10.1097/JTO.0000000000000630
21.
Bennett JA Braga AC Pinto A Van de Vijver K Cornejo K Pesci A et al Uterine PEComas: A morphologic, immunohistochemical, and molecular analysis of 32 tumors. Am J Surg Pathol (2018) 42(10):1370–83. 10.1097/PAS.0000000000001119
22.
Baniak N Barletta JA Hirsch MS . Key renal neoplasms with a female predominance. Adv Anat Pathol (2021) 28(4):228–50. 10.1097/PAP.0000000000000301
23.
Jenei A Hes O Kuthi L . Provisional renal cell carcinoma subsets following the 2016 WHO classification. Orv Hetil (2020) 161(3):83–94. 10.1556/650.2020.31654
24.
Yanai H Sasagawa I Kubota Y Ishigooka M Hashimoto T Kaneko H et al Spontaneous hemorrhage during pregnancy secondary to renal angiomyolipoma. Urol Int (1996) 56(3):188–91. 10.1159/000282838
25.
Oesterling JE Fishman EK Goldman SM Marshall FF . The management of renal angiomyolipoma. J Urol (1986) 135(6):1121–4. 10.1016/s0022-5347(17)46013-7
26.
Bakshi SS Vishal K Kalia V Gill JS . Aggressive renal angiomyolipoma extending into the renal vein and inferior vena cava - an uncommon entity. Br J Radiol (2011) 84(1004):e166–8. 10.1259/bjr/98449202
27.
Tong YC Chieng PU Tsai TC Lin SN . Renal angiomyolipoma: Report of 24 cases. Br J Urol (1990) 66(6):585–9. 10.1111/j.1464-410x.1990.tb07187.x
28.
Song S Park BK Park JJ . New radiologic classification of renal angiomyolipomas. Eur J Radiol (2016) 85(10):1835–42. 10.1016/j.ejrad.2016.08.012
29.
Park BK . Renal angiomyolipoma: Radiologic classification and imaging features according to the amount of fat. Am J Roentgenol (2017) 209(4):826–35. 10.2214/AJR.17.17973
30.
Shetty AS Sipe AL Zulfiqar M Tsai R Raptis DA Raptis CA et al In-phase and opposed-phase imaging: Applications of chemical shift and magnetic susceptibility in the chest and abdomen. Radiographics (2019) 39(1):115–35. 10.1148/rg.2019180043
31.
Shirotake S Yoshimura I Kosaka T Matsuzaki S . A case of angiomyolipoma of the renal sinus. Clin Exp Nephrol (2011) 15(6):953–6. 10.1007/s10157-011-0519-9
32.
Lin WY Chuang CK Ng KF Liao SK . Renal angiomyolipoma with lymph node involvement: A case report and literature review. Chang Gung Med J (2003) 26(8):607.
33.
Ackerman TE Levi CS Lindsay DJ Greenberg HM Gough JC . Angiomyolipoma with lymph node involvement. Can Assoc Radiol J (1994) 45(1):52.
34.
Stone CH Lee MW Amin MB Yaziji H Gown AM Ro JY et al Renal angiomyolipoma: Further immunophenotypic characterization of an expanding morphologic spectrum. Arch Pathol Lab Med (2001) 125(6):751–8. 10.1043/0003-9985(2001)125<0751:RA>2.0.CO;2
35.
Sooriakumaran P Gibbs P Coughlin G Attard V Elmslie F Kingswood C et al Angiomyolipomata: Challenges, solutions, and future prospects based on over 100 cases treated. BJU Int (2010) 105(1):101–6. 10.1111/j.1464-410X.2009.08649.x
36.
Schieda N Kielar AZ Al Dandan O McInnes MD Flood TA . Ten uncommon and unusual variants of renal angiomyolipoma (AML): Radiologic-pathologic correlation. Clin Radiol (2015) 70(2):206–20. 10.1016/j.crad.2014.10.001
37.
Aydin H Magi-Galluzzi C Lane BR Sercia L Lopez JI Rini BI et al Renal angiomyolipoma: Clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol (2009) 33(2):289–97. 10.1097/PAS.0b013e31817ed7a6
38.
Katabathina VS Vikram R Nagar AM Tamboli P Menias CO Prasad SR . Mesenchymal neoplasms of the kidney in adults: Imaging spectrum with radiologic-pathologic correlation. Radiographics (2010) 30(6):1525–40. 10.1148/rg.306105517
39.
Di BA Ferrara G Gogglia P . Renal capsuloma: Description of a case with predominantly muscular differentiation. Pathologica (2003) 95(2):119.
40.
Aldughiman AW Alzahrani A Alzahrani T . Renal leiomyoma: Case report and literature review. J Endourol Case Rep (2019) 5(4):181–3. 10.1089/cren.2019.0049
41.
Gobbo S Eble JN Huang J Grignon DJ Wang M Martignoni G et al Schwannoma of the kidney. Mod Pathol (2008) 21(6):779–83. 10.1038/modpathol.2008.52
42.
Mehta V Venkataraman G Antic T Rubinas TC Le Poole IC Picken MM . Renal angiomyolipoma, fat-poor variant - a clinicopathologic mimicker of malignancy. Virchows Arch (2013) 463(1):41–6. 10.1007/s00428-013-1432-2
43.
Oh SD Oh SJ Suh BJ Shin JY Oh CK Park JK et al A giant retroperitoneal liposarcoma encasing the entire left kidney and adherent to adjacent structures: A case report. Case Rep Oncol (2016) 9(2):368–72. 10.1159/000447488
44.
Knebel C Neumann J Schwaiger BJ Karampinos DC Pfeiffer D Specht K et al Differentiating atypical lipomatous tumors from lipomas with magnetic resonance imaging: A comparison with MDM2 gene amplification status. BMC Cancer (2019) 19(1):309. 10.1186/s12885-019-5524-5
45.
Davis CJ Barton JH Sesterhenn IA . Cystic angiomyolipoma of the kidney: A clinicopathologic description of 11 cases. Mod Pathol (2006) 19(5):669–74. 10.1038/modpathol.3800572
46.
Fine SW Reuter VE Epstein JI Argani P . Angiomyolipoma with epithelial cysts (AMLEC): A distinct cystic variant of angiomyolipoma. Am J Surg Pathol (2006) 30(5):593–9. 10.1097/01.pas.0000194298.19839.b4
47.
Armah HB Yin M Rao UN Parwani AV . Angiomyolipoma with epithelial cysts (AMLEC): A rare but distinct variant of angiomyolipoma. Diagn Pathol (2007) 2:11. 10.1186/1746-1596-2-11
48.
Michal M Hes O Bisceglia M Simpson RH Spagnolo DV Parma A et al Mixed epithelial and stromal tumors of the kidney. A report of 22 cases. Virchows Arch (2004) 445(4):359–67. 10.1007/s00428-004-1060-y
49.
Nese N Martignoni G Fletcher CD Gupta R Pan CC Kim H et al Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: Detailed assessment of morphology and risk stratification. Am J Surg Pathol (2011) 35(2):161–76. 10.1097/PAS.0b013e318206f2a9
50.
Brimo F Robinson B Guo C Zhou M Latour M Epstein JI . Renal epithelioid angiomyolipoma with atypia: A series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol (2010) 34(5):715–22. 10.1097/PAS.0b013e3181d90370
51.
He W Cheville JC Sadow PM Gopalan A Fine SW Al-Ahmadie HA et al Epithelioid angiomyolipoma of the kidney: Pathological features and clinical outcome in a series of consecutively resected tumors. Mod Pathol (2013) 26(10):1355–64. 10.1038/modpathol.2013.72
52.
Mete O van der Kwast TH . Epithelioid angiomyolipoma: A morphologically distinct variant that mimics a variety of intra-abdominal neoplasms. Arch Pathol Lab Med (2011) 135(5):665–70. 10.1043/2009-0637-RSR.1
53.
Giannikou K Malinowska IA Pugh TJ Yan R Tseng YY Oh C et al Whole exome sequencing identifies TSC1/TSC2 biallelic loss as the primary and sufficient driver event for renal angiomyolipoma development. Plos Genet (2016) 12(8):e1006242. 10.1371/journal.pgen.1006242
54.
Li W Guo L Bi X Ma J Zheng S . Immunohistochemistry of p53 and Ki-67 and p53 mutation analysis in renal epithelioid angiomyolipoma. Int J Clin Exp Pathol (2015) 8(8):9446.
55.
Roma AA Magi-Galluzzi C Zhou M . Differential expression of melanocytic markers in myoid, lipomatous, and vascular components of renal angiomyolipomas. Arch Pathol Lab Med (2007) 131(1):122–5. 10.1043/1543-2165(2007)131[122:DEOMMI]2.0.CO;2
56.
Bonsib SM Boils C Gokden N Grignon D Gu X Higgins JP et al Tuberous sclerosis complex: Hamartin and tuberin expression in renal cysts and its discordant expression in renal neoplasms. Pathol Res Pract (2016) 212(11):972–9. 10.1016/j.prp.2016.04.005
57.
Sobiborowicz A Czarnecka AM Szumera-Ciećkiewicz A Rutkowski P Świtaj T . Rozpoznanie i leczenie nowotworów typu angiomyolipoma (AML). Oncol Clin Pract (2020) 16(3):116–32. 10.5603/ocp.2020.0008
58.
Ljungberg B Albiges L Abu-Ghanem Y Bensalah K Dabestani S Fernández-Pello S et al European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol (2019) 75(5):799–810. 10.1016/j.eururo.2019.02.011
59.
Fernández-Pello S Hora M Kuusk T Tahbaz R Dabestani S Abu-Ghanem Y et al Management of sporadic renal angiomyolipomas: A systematic review of available evidence to guide recommendations from the European association of urology renal cell carcinoma guidelines panel. Eur Urol Oncol (2020) 3(1):57–72. 10.1016/j.euo.2019.04.005
60.
Vos N Oyen R . Renal angiomyolipoma: The good, the bad, and the ugly. J Belg Soc Radiol (2018) 102(1):41. 10.5334/jbsr.1536
61.
Kuusk T Biancari F Lane B Tobert C Campbell S Rimon U et al Treatment of renal angiomyolipoma: Pooled analysis of individual patient data. BMC Urol (2015) 15:123. 10.1186/s12894-015-0118-2
62.
Bissler JJ Kingswood JC . Renal angiomyolipomata. Kidney Int (2004) 66(3):924–34. 10.1111/j.1523-1755.2004.00838.x
63.
Lei JH Liu LR Wei Q Song TR Yang L Yuan HC et al A four-year follow-up study of renal epithelioid angiomyolipoma: A multi-center experience and literature review. Sci Rep (2015) 5:10030. 10.1038/srep10030
64.
Lattanzi M Deng FM Chiriboga LA Femia AN Meehan SA Iyer G et al Durable response to anti-PD-1 immunotherapy in epithelioid angiomyolipoma: A report on the successful treatment of a rare malignancy. J Immunother Cancer (2018) 6(1):97. 10.1186/s40425-018-0415-x
Summary
Keywords
AML, angiomyolipoma, kidney tumor, tuberous sclerosis, nephrectomy
Citation
Fejes Z, Sánta F, Jenei A, Király IE, Varga L and Kuthi L (2023) Angiomyolipoma of the kidney—Clinicopathological analysis of 52 cases. Pathol. Oncol. Res. 28:1610831. doi: 10.3389/pore.2022.1610831
Received
19 September 2022
Accepted
20 December 2022
Published
09 January 2023
Volume
28 - 2023
Edited by
József Tímár, Semmelweis University, Hungary
Updates
Copyright
© 2023 Fejes, Sánta, Jenei, Király, Varga and Kuthi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Levente Kuthi, kuthi.levente@med.u-szeged.hu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.