Abstract
Background: SO (Struma ovarii) is a rare form of ovarian teratoma which originates from ovarian dermoid cysts. Due to the rarity of this disease, relevant studies might not be sufficiently documented, especially cases with hyperthyroidism and multiple metastases.
Case Presentation: A 40-year-old female patient was admitted to our hospital due to management of early pregnancy along with a recurrent abdominal and pelvic mass. Contrast-enhanced CT images showed an irregular mass (10.7 × 8.6 × 12.8 cm) located in the right side from the hypogastrium to the pelvic cavity and another mass (3.8 × 3.7 cm) in the liver. Laboratory examination showed that CA125 (Carbohydrate Antigen-125) was 118.10 U/mL, Tg (thyroglobulin) was >300 ng/ml, FT4 (free thyroxine) was 22.11 pmol/L, and TSH (thyroid-stimulating hormone) was <0.004 mIU/L. She subsequently underwent liver mass dissection, omentectomy, tumor dissection, peritoneal nodule resection, as well as rectal anterior wall nodule resection. The patient was diagnosed with malignant SO (papillary type) along with multiple metastases. Also, we conducted a literature review based on 290 SO cases from 257 articles.
Conclusion: This study showed that malignant SO might be prone to relapse and metastasize (a metastatic rate of 52.94%) and therefore aggressive management might need to be recommended for malignant SO. Also, laparotomy might need to be recommended for large tumors that cannot be resected by laparoscopic surgery since these tumors might be prone to rupture and thus produce peritoneal implants. Furthermore, Graves’ disease might need to be considered in the differential diagnosis.
Introduction
SO (Struma ovarii), a rare form of ovarian teratoma which originates (more than 50%) from ovarian dermoid cysts consisting of thyroid tissue, accounts for about 1% of all ovarian neoplasms and about 2% of mature ovarian teratomas (1, 2). According to several reports, the age at diagnosis ranged largely from 12 to 77 years of age with a median age of 43 (3, 4). Due to the rarity of this disease, relevant studies might not be sufficiently documented. Thus, the prognosis and modalities of treatment are still somewhat controversial. This case report presented a rare diagnosis of malignant SO with hyperthyroidism and multiple metastases.
The differential diagnosis between malignant SO and benign SO varied largely over the years. Some researchers hypothesize that malignancy can only be diagnosed when the tumor shows definite invasion or metastases, while others diagnose malignancy on nuclear or histologic alterations (5). In most instances, the diagnosis of malignant SO is only made postoperatively (6). As a result, some cases might have been misdiagnosed as benign SO. At the same time, due to the rarity of this disease, an approved consensus on the diagnosis of benign and malignant SO has not been arrived at yet. In this study, we also retrieved a total of 290 cases from 257 articles aiming to find and discuss more disparities that might be helpful in the differential diagnosis between these two forms of disease.
Case Presentation
A 30-year-old female with early pregnancy and a left ovarian mass was admitted to a local primary hospital, where she later received a laparoscopic mass dissection and induced abortion in January 2008. Postoperative histology suggested the presence of an inclusion cyst. In August 2008, the patient was readmitted to that hospital due to a right ovarian mass with the greatest dimension up to 10 cm. Then, the patient received another laparoscopic resection of the mass which ruptured in the pelvic cavity during the dissection. Postoperative histology confirmed that the mass was composed of SO (unknown histology, unknown if malignant). The patient did not have any follow-up until 5 years later when a new pelvic cystic mass (4.0 cm in greatest dimension) was discovered. However, she declined treatment. Then in April 2018, at the age of 40, the patient was admitted to the obstetrics and gynecology department of our hospital for management of another early pregnancy along with a recurrent abdominal and pelvic mass which had enlarged from 4.0 to 9.0 cm.
During the physical examination, there were no remarkable findings, apart from a palpable mass in the right lower abdomen with no tenderness or rebound tenderness.
Contrast-enhanced CT (computed tomography) images of the whole abdomen and pelvic cavity showed an irregular mass (10.7 × 8.6 × 12.8 cm) located in the right side from the hypogastrium to the pelvic cavity containing both solid and cystic components with regional irregular calcifications. The mass was surrounded by multiple enhancing lesions and low-density nodules of different sizes with the largest one up to 4.3 cm in diameter (Figure 1A). In addition, a similar enhancing mass (3.8 × 3.7 cm) was also found in the Couinaud’s S6 segment of the liver (Figure 1B). Gynecological ultrasonography confirmed the abdomino-pelvic mass which contained solid and cystic components. Doppler vascular flow was noted only in the solid component. No abnormality was detected on ultrasonography of the thyroid gland and cervical lymph nodes. Laboratory examination showed that CA125 (Carbohydrate Antigen-125) was 118.10 U/mL (reference range: 0.00–35.00 U/mL), Tg (thyroglobulin) was >300 ng/ml (reference range: 0.00–55.00 ng/ml), FT4 (free thyroxine) was 22.11 pmol/L (reference range: 9.01–19.05 pmol/L), and TSH (thyroid-stimulating hormone) was <0.004 mIU/L (reference range: 0.350–4.940 mIU/L). Carcinoembryonic antigen, alpha-fetoprotein, anti-thyroglobulin antibody as well as calcitonin were all within the normal range. The patient later opted for a surgical abortion to further remove the abdominal and pelvic mass. In May 2018, the patient was transferred to the general surgical department of our hospital to receive surgery.
FIGURE 1
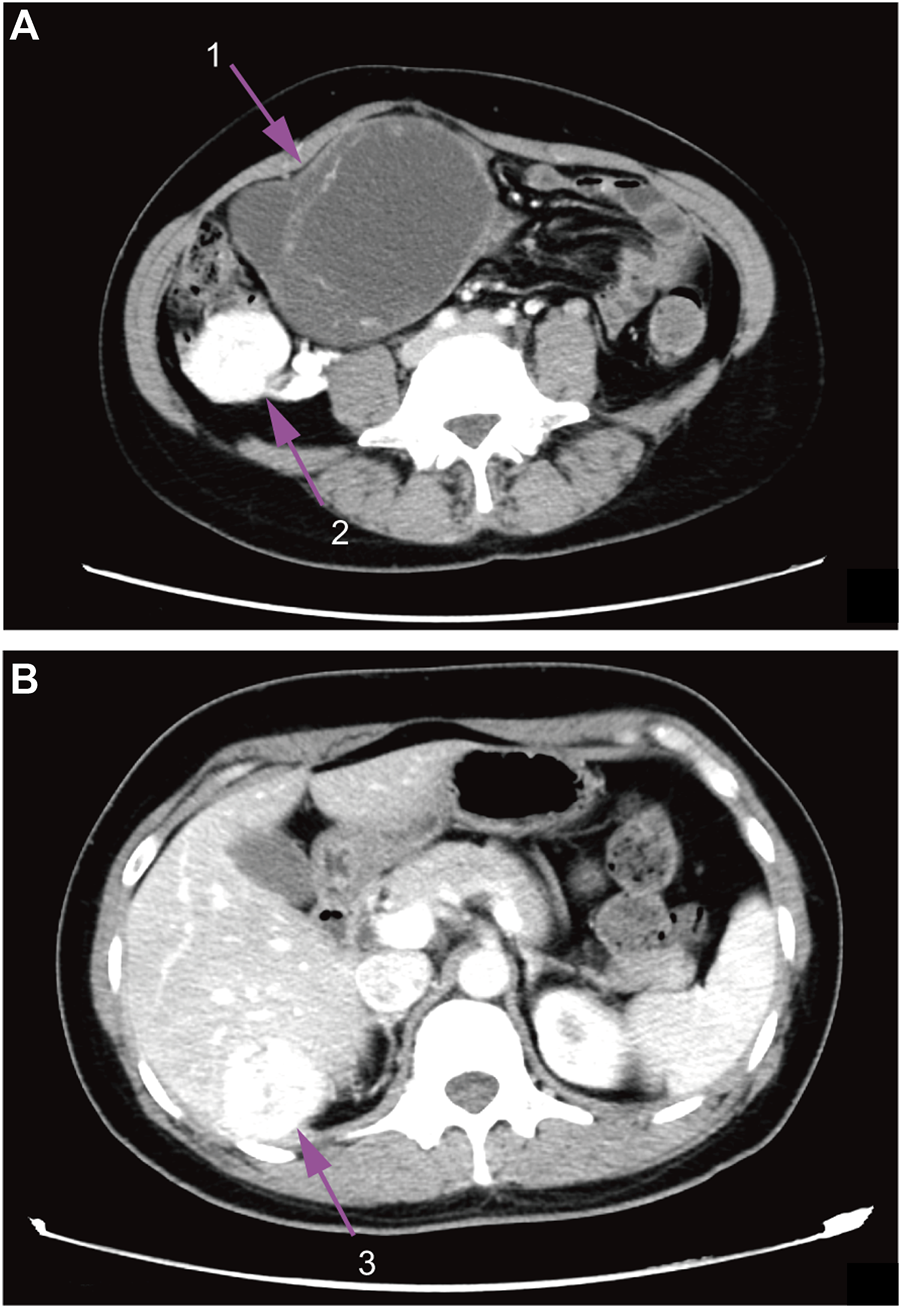
Contrast-enhanced CT images. (A), abdomen image. (B), pelvic cavity image; 1, ovarian mass; 2, surrounding lesions of the ovarian mass; 3, enhancing liver mass.
Laparoscopy showed multiple light red nodules which were each about 1.0 cm in diameter in the greater omentum and the right side of the peritoneum, one large exophytic mass (5.0 cm in diameter) with a grey surface in the S6 segment of the liver (without invasion of the liver parenchyma), as well as a large polycystic mass in the right adnexal region with a high-surface-tension and a complete capsule. No ascites had formed in the patient’s abdomen. A diagnostic resection of the peritoneal nodules was performed using an ultrasonic scalpel and tissues were immediately sent for rapid pathological examination. It showed that components of thyroid tissue could be found in the nodules and thus they were considered to be metastatic lesions that might arise from the SO. Subsequently, a liver mass dissection and omentectomy were performed in sequence. Laparoscopic instruments were then withdrawn. Laparotomy was immediately performed. During the laparotomy, total tumor dissection, unilateral salpingo-oophorectomy, peritoneal nodule resection, as well as rectal anterior wall nodule resection were performed. Due to the patient’s strong will of preserving her fertility, the uterus and the left ovary were preserved.
Pathologic examination after the surgery indicated that the thyroid component could be found in the tissues removed from the right ovary. Hematoxylin-eosin (HE) staining showed that there was a presence of papillae. Irregular, enlarged, ground glass, and overlapping nuclei with grooves and pseudoinclusions could also be found after staining. The tumor was entirely composed of the above component and there was no evidence of benign SO. Immunohistochemically, Galectin-3, CK19 (cytokeratin-19), TTF-1 (thyroid transcription factor-1), and Ki-67 had positive expressions (Figure 2). Tissues of the liver mass, rectal wall nodules, and peritoneal nodules had the same nuclear features as those of the SO (Supplementary Figure S1). Immunohistochemically, these tissues had exactly the same expressions as those of the SO. Also, no abnormality was detected in the thyroid gland and therefore they were considered to be metastatic lesions originated from the SO. Based on findings of HE staining and immunohistochemical staining, as well as clinical recurrence of SO and multiple metastases, the patient was diagnosed with malignant SO (papillary type).
FIGURE 2
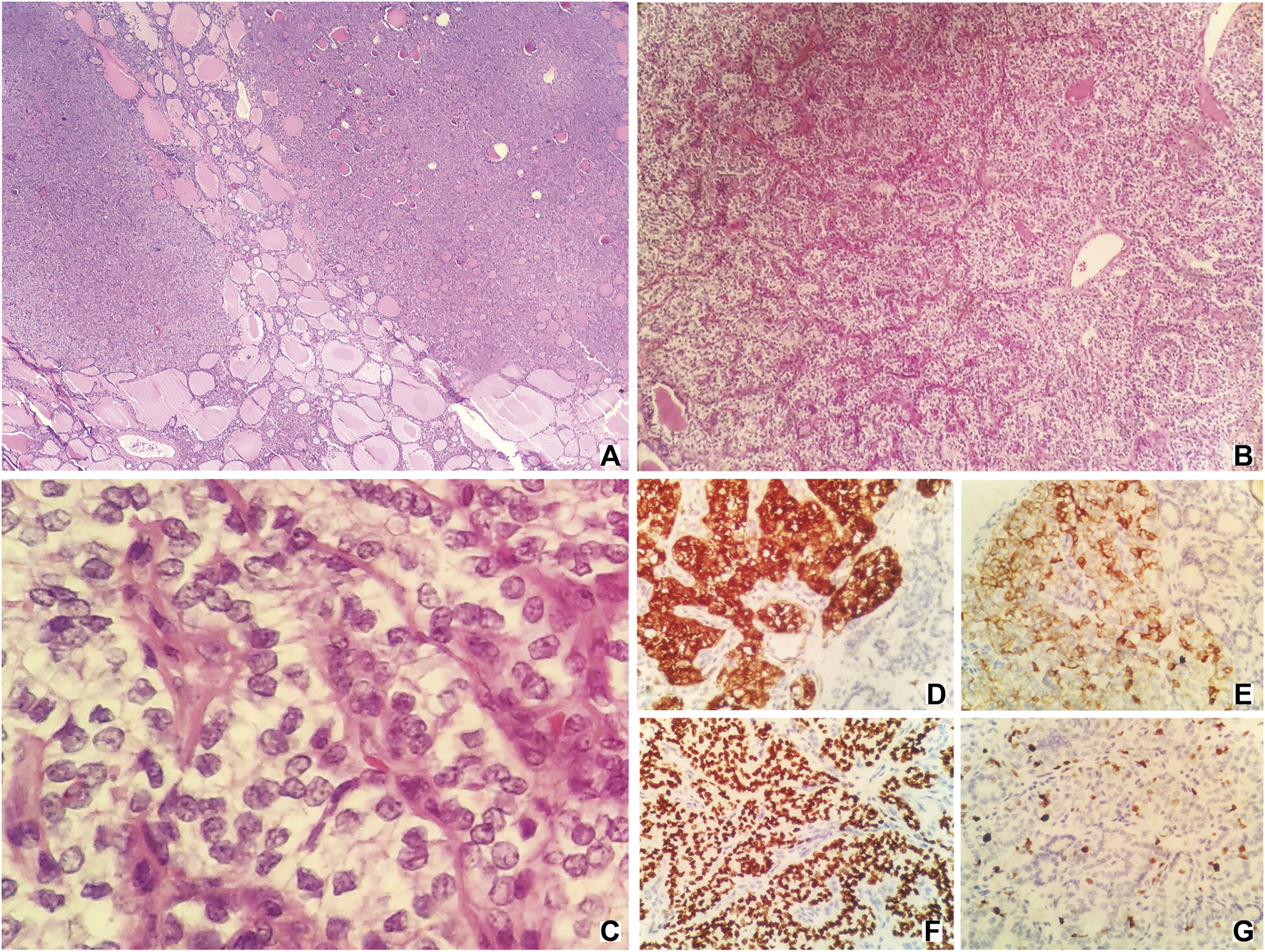
HE staining [(A–C); original magnification ×40, 100, and 400, respectively] and immunohistochemical staining [(D–G); original magnification ×400] of samples taken from the tumor. HE staining (A,B) showing the presence of papillae. HE staining c showing irregular, enlarged, ground glass, and overlapping nuclei with grooves, and pseudoinclusions. (D), Galectin-3; (E), CK19; (F), TTF-1; (G), Ki-67.
A laboratory test was performed at 1-month follow-up after the surgery and it showed a normal level of FT4 (11.28 pmol/L), TSH (2.518 mIU/L), and Tg (26.50 ng/ml, timeline with relevant serum indicators seen in Supplementary Table S1). In November 2018, 6 months after the laparotomy, a follow-up contrast-enhanced CT examination showed another mass with mixed density and irregular enhancement in the left adnexal region (4.7 × 2.4 cm). Thyroid hormones remained normal. However, the patient refused to accept any other treatment and was since lost to follow up.
Literature Review
Materials and Methods
A PubMed literature search of SO case report was performed, with the use of the following terms: “struma ovarii,” “malignant struma ovarii,” and “benign struma ovarii.” A total of 484 articles were retrieved in the search. After selection, 257 eligible articles containing 290 eligible cases were reviewed (including 99 cases with unknown histological behavior, 114 malignant SO cases accounting for 59.69%, and 77 benign SO cases accounting for 40.31%). The search criteria were shown in Supplementary Figure S2. Each variable was stratified by histological behavior. Two-tailed Chi-square test (for discrete variables), two-tailed t test (for continuous variables with a normal distribution), as well as two-tailed K-W test (Kruskal-Wallis test, for continuous variables with a nonnormal distribution) were performed to compare the difference between malignant SO and benign SO. A p value <0.05 was considered to be statistically significant. Because there was a third category of SO with unknown histological behavior that could not be classified as benign or malignant, these cases were excluded from further analyses. Also, there were cases with unknown or N/A (not applicable) patient data and these cases were excluded from further analyses to ensure the accuracy of this study.
Results
As shown in Table 1, SO occurred with onset around the age of 47.38 (±16.12) overall. No significant statistical difference was found in terms of age between malignant SO and benign SO (p value = 0.292, Figure 3A). The incidence of tumors arising from the right ovary was slightly higher than that arising from the left whether for malignant SO, benign SO, or unknown histological behavior SO, but there was no significant statistical difference between malignant SO and benign SO (p value = 0.410). Also, no significant statistical difference was found in tumor size between these two groups (p value = 0.474, Figure 3B). For histology, papillary type accounted for the majority of malignant SO (47.62%), whereas follicular type accounted for the majority of benign SO (51.61%) (p value = 0.002). For clinical symptoms, no significant statistical differences were found whether in abdominal discomfort, abdominal genital bleeding, or urinary compressive symptom between malignant SO and benign SO (all p values > 0.05), but the occurrence of hyperthyroidism was higher for benign SO than that of malignant SO (39.02% vs. 11.94%, p value = 0.001). For serum tumor marker CA125, more positive cases were observed in benign SO with a higher serum level than those of malignant SO (all p values < 0.05, Table 1; Figure 3C). As for tumor marker Tg, no significant statistical differences were found whether in terms of the positivity rate (percentage of elevated Tg in all malignant or benign SO cases) or serum level between these two groups (all p values > 0.05, Table 1; Figure 3D). For the application of surgical methods including salpingo-oophorectomy, pelvic washing, total hysterectomy, and omentectomy, no statistical difference was found between malignant SO and benign SO although benign SO had higher percentages in terms of more aggressive surgical methods. However, more malignant SO cases received total thyroidectomy and RAI (radioactive iodine) treatment than those with benign SO (59.43% vs. 10.71% for total thyroidectomy, 58.04% vs. 11.11% for RAI, all p values <0.001). Malignant SO had a higher rate of metastasis than that of benign SO and there was a significant statistical difference (52.94% vs. 15.38%, p value = 0.001).
TABLE 1
| Malignant (%) | Benign (%) | Unknown Behavior (%) | Total (%) | p value | |
|---|---|---|---|---|---|
| Age | 45.21 ± 14.30 | 47.74 ± 17.40 | 49.59 ± 16.88 | 47.38 ± 16.12 | 0.292 |
| Tumor location | 0.410 | ||||
| Left ovary | 51 (48.11) | 32 (42.11) | 34 (37.78) | 117 (43.01) | |
| Right ovary | 55 (51.89) | 43 (56.58) | 52 (57.78) | 150 (55.15) | |
| Both ovaries | 0 (0) | 1 (1.32) | 4 (4.44) | 5 (1.84) | |
| Unknown | 8 | 1 | 9 | 18 | |
| Histology | 0.002 | ||||
| Papillary | 50 (47.62) | 6 (19.35) | 23 (38.98) | 79 (40.51) | |
| Follicular | 27 (25.71) | 16 (51.61) | 17 (28.81) | 60 (30.77) | |
| Follicular variant papillary | 21 (20.00) | 3 (9.68) | 6 (10.17) | 30 (15.38) | |
| Other | 7 (6.67) | 6 (19.35) | 13 (22.03) | 26 (13.33) | |
| Unknown | 9 | 46 | 40 | 95 | |
| Abdominal discomfort | 0.267 | ||||
| Yes | 55 (55.00) | 30 (46.15) | 37 (45.68) | 122 (49.59) | |
| No | 45 (45.00) | 35 (53.85) | 44 (54.32) | 124 (50.41) | |
| Unknown | 14 | 12 | 18 | 44 | |
| Abnormal genital bleeding | 0.149 | ||||
| Yes | 7 (7.00) | 1 (1.54) | 8 (9.88) | 16 (6.50) | |
| No | 93 (93.00) | 64 (98.46) | 73 (90.12) | 230 (93.50) | |
| Unknown | 14 | 12 | 18 | 44 | |
| Urinary compressive symptom | 1.000 | ||||
| Yes | 3 (2.97) | 2 (3.08) | 3 (3.70) | 8 (3.24) | |
| No | 98 (97.03) | 63 (96.92) | 78 (96.30) | 239 (96.76) | |
| Unknown | 13 | 12 | 18 | 43 | |
| Hyperthyroidism | 0.001 | ||||
| Yes | 8 (11.94) | 16 (39.02) | 15 (31.91) | 39 (25.16) | |
| No | 59 (88.06) | 25 (60.98) | 32 (68.09) | 116 (74.84) | |
| Unknown | 47 | 36 | 52 | 135 | |
| Serum CA125 | 0.001 | ||||
| Positive | 16 (42.11) | 34 (79.07) | 13 (44.83) | 63 (57.27) | |
| Negative | 22 (57.89) | 9 (20.93) | 16 (55.17) | 47 (42.73) | |
| Unknown | 76 | 34 | 70 | 180 | |
| Serum Tg | 0.867 | ||||
| Positive | 29 (51.79) | 6 (54.55) | 15 (75.00) | 50 (57.47) | |
| Negative | 27 (48.21) | 5 (45.45) | 5 (25.00) | 37 (42.53) | |
| Unknown | 58 | 66 | 79 | 203 | |
| Salpingo-oophorectomy | 0.093 | ||||
| Unilateral | 39 (41.05) | 19 (32.20) | 24 (31.58) | 82 (35.65) | |
| Bilateral | 50 (52.63) | 30 (50.85) | 38 (50.00) | 118 (51.30) | |
| None | 6 (6.32) | 10 (16.95) | 14 (18.42) | 30 (13.04) | |
| Unknown | 7 | 16 | 18 | 41 | |
| N/A | 12 | 2 | 5 | 19 | |
| Pelvic washing | 0.792 | ||||
| Yes | 7 (6.67) | 3 (4.48) | 3 (3.66) | 13 (5.12) | |
| No | 98 (93.33) | 64 (95.52) | 79 (96.34) | 241 (94.88) | |
| Unknown | 9 | 10 | 17 | 36 | |
| Total hysterectomy | 0.331 | ||||
| Yes | 55 (52.38) | 30 (44.78) | 31 (38.75) | 116 (46.03) | |
| No | 50 (47.62) | 37 (55.22) | 49 (61.25) | 136 (53.97) | |
| Unknown | 7 | 10 | 16 | 33 | |
| N/A | 2 | — | 3 | 5 | |
| Omentectomy | 0.268 | ||||
| Yes | 35 (33.33) | 17 (25.37) | 13 (15.85) | 65 (25.59) | |
| No | 70 (66.67) | 50 (74.63) | 69 (84.15) | 189 (74.41) | |
| Unknown | 9 | 10 | 17 | 36 | |
| Total thyroidectomy | <0.001 | ||||
| Yes | 63 (59.43) | 6 (10.71) | 20 (24.69) | 89 (36.63) | |
| No | 43 (40.57) | 50 (89.29) | 61 (75.31) | 154 (63.37) | |
| Unknown | 2 | 10 | 13 | 25 | |
| N/A | 6 | 11 | 5 | 22 | |
| RAI treatment | <0.001 | ||||
| Yes | 65 (58.04) | 7 (11.11) | 18 (22.22) | 90 (35.16) | |
| No | 47 (41.96) | 56 (88.89) | 63 (77.78) | 166 (64.84) | |
| Unknown | 1 | 10 | 15 | 26 | |
| N/A | 1 | 4 | 3 | 8 | |
| Metastasis | 0.001 | ||||
| Yes | 45 (52.94) | 4 (15.38) | 17 (43.59) | 66 (44.00) | |
| No | 40 (47.06) | 22 (84.62) | 22 (56.41) | 84 (56.00) | |
| Unknown | 29 | 49 | 60 | 138 | |
| N/A | — | 2 | — | 2 |
Description of the study population.
N/A, not applicable; CA125, carbohydrate Antigen-125; Tg, thyroglobulin; RAI, radioactive iodine; %, percentage (continuous variables not included); Unknown histological behaviors and unknown or N/A cases of all variables not included in the t test or Chi-square test.
FIGURE 3
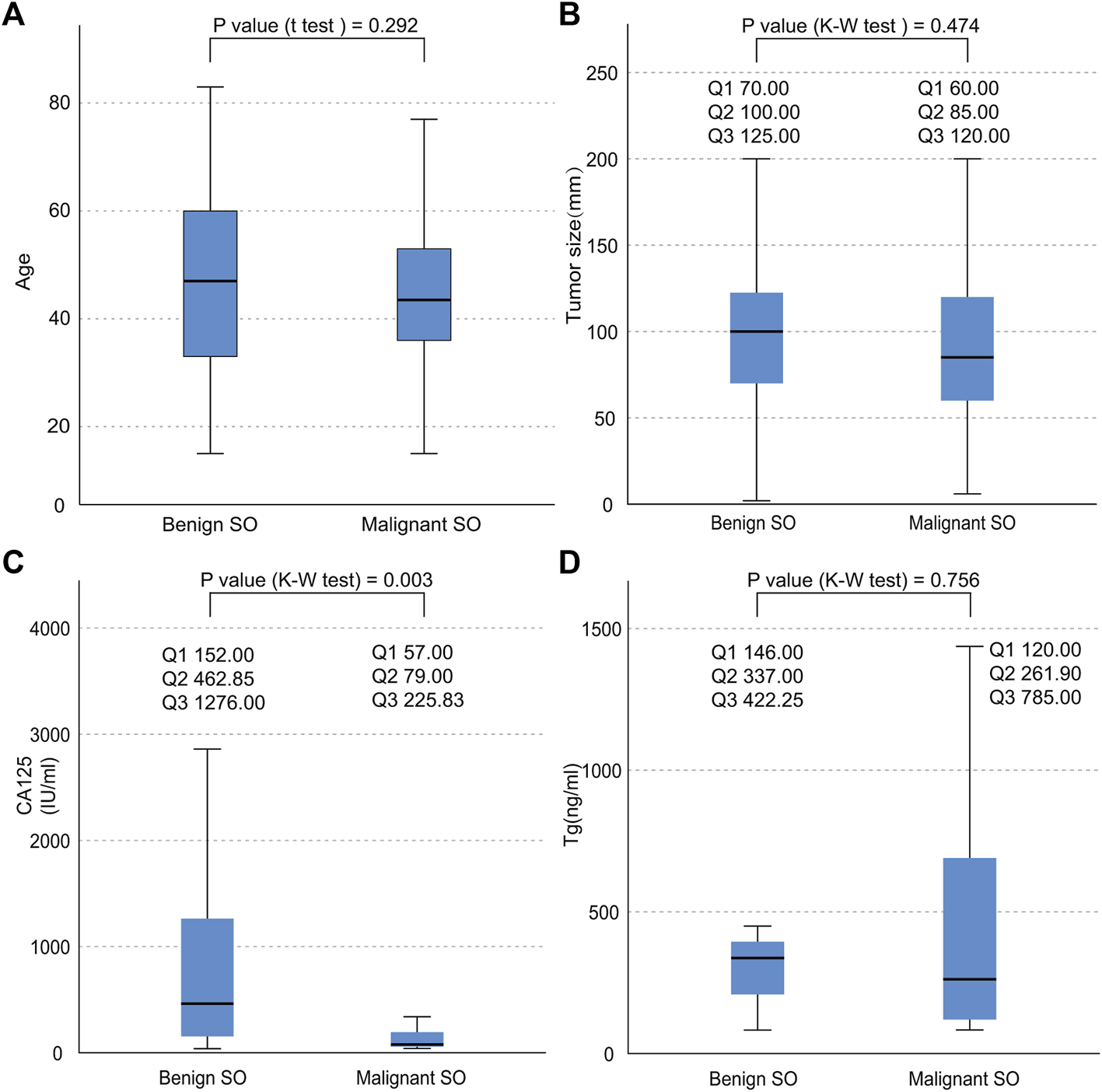
Data distribution of the continuous variables of both benign SO and malignant SO. (A), age distribution; (B), tumor size distribution; (C), serum CA125 distribution; (D), serum Tg distribution; nonnormal variables stated as quartiles; Q1, first quartile; Q2, second quartile; Q3, third quartile.
Discussion
Mature teratomas are composed of tissues from ectodermal, mesodermal, and endodermal origins (7). SO, originated from primordial germ cells of the ovary, is a monodermal and highly specialized mature teratoma and is composed almost entirely of mature thyroid tissue which shows acini filled with thyroid colloid (8). SO includes two forms of histological behavior- malignant and benign. Malignant SO has the same molecular mechanism including BRAF and RAS point mutation and RET/PTC gene rearrangement as that of malignant tumors which originate from the thyroid gland (9, 10). About 5%–15% of ovarian teratomas contain thyroid tissue and they are not necessarily SO. The difference between them is that SO is diagnosed when thyroid tissue is the predominant element. SO, as a variant of ovarian teratoma only accounts for 2% of all ovarian teratomas with thyroid tissue, and approximately only 0.1%–5% of all SO are considered as malignant (11–14). Our study showed that malignant SO accounted for the majority of all SO cases (59.69%) and this result contradicted previous studies. One possible reason is that benign SO is a less rare disease compared with malignant SO, and thus it has been rarely reported. Another, more likely explanation, is that given the lack of clinical complications from benign SO, large series of benign SO are not reported as often as malignant series.
The currently-accepted diagnostic criteria of SO were the one summarized by K Devaney et al. as follows (15, 16): first, the ovarian thyroid tissue must contain cytologically malignant cells and the presence of certain invasion may also contribute to the diagnosis; second, histopathologically, it must exhibit a similar type as that of papillary or follicular carcinoma, sometimes oncocytic or anaplastic carcinoma; third, an immunohistochemically positive Tg expression is also helpful. For papillary type, the diagnosis depends on the appearance of papilla, invasion, and ground glass, empty or overlapping nuclei of the cells, and sometimes along with the existence of psammoma bodies in the tissue.
There has always been controversy over whether cases initially diagnosed as “benign” or “unknown behavior” which developed distant metastases should be classified as malignant SO. Some researchers suggested that malignancy can only be diagnosed when the tumor shows definite invasion or metastases (12, 17), while others diagnose malignancy on nuclear or histologic alterations (including grooves and ground-glass appearance with papillary architecture) (6, 15). Lack of consensus strongly imposes difficulties for diagnosis under this circumstance. Apart from the presence of extraovarian spread, recurrence after initial surgery is another important indication in terms of differential diagnosis, as Shaco-Levy et al. described in their research (18). They subsequently performed another large, blinded study of 86 SO cases aiming to distinguish the pathological disparities between malignant and benign SO. Interestingly, the authors concluded that most pathological features (e.g., papillae, psammoma bodies, nuclear grooves, nuclear overlap, etc) did not correlate with biological behaviors (19). That is, if confined to the ovarian or temporarily “exempted from” relapse, these tumors will not be correctly identified. We agreed that both extraovarian development and recurrence might be more indicative. However, as mentioned above, the establishment of relevant guidelines will surely be needed. On the other hand, we speculated that immunohistochemical findings might also be potentially helpful. For example, some researchers suggested that a positive expression of CK19 is also highly supportive in the diagnosis of malignancy (20), similar to the case in this study. Overall, since this patient showed all the above characteristics, she was diagnosed with malignant SO.
Our study also showed that in general, papillary accounted for the majority of all histological types (40.51%). The study of Wei, S et al., also showed that papillary type was the commonest well-differentiated neoplasm arising in SO (21). This clinical characteristic was similar to that of thyroid carcinoma. However, our study showed that when stratified by histological behavior, papillary type accounted for the majority of malignant SO (47.62%), whereas follicular type accounted for the majority of benign SO (51.61%, Table 1).
Clinical characteristics of SO may include lower abdominal discomfort, abnormal vaginal bleeding and some of those cases may also present ascites, hydrothorax, or hyperthyroidism (22, 23). Our study showed that there were no significant statistical differences in terms of clinical characteristics including abdominal discomfort, abnormal genital bleeding, urinary compressive symptoms between malignant SO and benign SO. Clinically, this might impose difficulties in the differential diagnosis.
According to several reports, only 8% of all cases presented with clinical hyperthyroidism (24, 25). Our study showed a much higher occurrence rate overall (25.16%, Table 1) and benign SO was more likely to develop hyperthyroidism compared with malignant SO (39.02% vs. 11.94%, p value = 0.001). We suspected that malignant SO is often poorly differentiated, and thus it rarely develops functioning thyroid tissue. In our case, despite the malignancy, laboratory examination also showed hyperthyroidism. Since no abnormality was detected in ultrasonography when examining the thyroid gland and both FT4 and TSH levels became normal after the surgery, the hyperthyroidism was likely caused by the hyperfunctioning SO (26, 27). Clinically, the diagnosis of SO might be masked by Graves’ disease.
This study showed that the positivity rate of CA125 was about 57.27% in all tumors (including malignant, benign and unknown behavior SO). This result was relatively consistent with previous studies which suggested that the positive value of CA125 was around 60% for all ovarian cancers (28, 29). This suggested that CA125 can not be used as a specific indicator for SO, but it can be helpful in the diagnosis, especially for benign SO with a relatively higher positivity rate (up to 79.07%) and serum level compared with those of malignant SO (Table 1; Figure 3C, all p values < 0.05).
Serum Tg may also be a useful indicator in terms of the diagnosis and monitoring of SO. Several studies suggested that the elevated Tg concentration was a useful indicator in the diagnosis and Tg level could be used for detecting the recurrence of SO for patients who received total thyroidectomy (30). Our case study also confirmed the value of this indicator.
Since malignant SO is an extremely rare clinical disease and only a few studies were documented, its therapeutic method has yet to be improved. The therapy, for the time being, ranges largely from radical surgery including total hysterectomy, bilateral salpingo-oophorectomy, and total thyroidectomy followed by postoperative RAI treatment to conservative management including unilateral oophorectomy or preservation of reproductive function and thyroid gland. Chemotherapy may also be useful (5, 31, 32). In our study, no statistical difference was found in terms of the application of salpingo-oophorectomy, pelvic washing, total hysterectomy, and omentectomy between malignant SO and benign SO although malignant SO had higher percentages in terms of more aggressive surgical methods. More malignant SO cases received total thyroidectomy and RAI treatment than those with benign SO. This might be explained by the consideration that malignant SO had a higher metastatic possibility than that of benign SO (52.94% vs 15.38%, p value = 0.001). The study of Marti JL, et al. also suggested that thyroidectomy with RAI therapy is reasonable when extra-ovarian spread, metastases, or synchronous primary thyroid gland cancer are present (33).
During the laparoscopic mass dissection in a local primary hospital in August 2008, the mass ruptured. Afterward, another pelvic mass was formed. We suspected that the occurrence of malignant SO might be peritoneal implants at that time, although we could not retrieve more details concerning the pathological confirmation from that surgery. Since malignant SO often has a thin capsule and a large size (first quartile: 60 mm, second quartile: 85 mm, third quartile: 120 mm, Figure 3B) and might be prone to rupture, this might need to be avoided during surgery.
A follow-up contrast-enhanced CT examination showed another recurrent mass (4.7 × 2.4 cm) with mixed density and irregular enhancement in the left adnexal region 6 months after the laparotomy. Although the histology could not be determined because the patient refused any other treatment, we suspected that this might be another recurrent SO. This case report and literature review might be helpful to some extent in the knowledge of this disease.
There were two limitations in this study. First, hyperthyroidism seen in this patient was diagnosed based on the fact that thyroid hormone and TSH levels normalized after resection. However, a 123I scan was not performed due to the concerns of avoiding overdiagnosis. Second, analytic results of the literature review only represent cases that have been published to date and they might change to some extent in the future when more cases are added.
Conclusion
This case report showed that the patient presented with SO repeatedly, which indicated that malignant SO might be prone to relapse. Besides, our literature review also showed that malignant SO often has a high metastatic rate (52.94%). These results suggested that aggressive management including total hysterectomy, bilateral salpingo-oophorectomy, and total thyroidectomy followed by postoperative RAI treatment might need to be recommended for malignant SO. Also, laparotomy might need to be recommended for large tumors that can not be resected by laparoscopic surgery since these tumors might be prone to rupture and thus produce peritoneal implants. Furthermore, a differential diagnosis of other causes of hyperthyroidism might need to be considered.
Patient Perspective
At 1 month follow-up after the laparotomy, the patient and her family were satisfied with the treatment effect.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board and Ethics Committee of Tianjin Medical University General Hospital (ethical approval number: IRB2021-004-01). The patients/participants provided their written informed consent to participate in this study.
Author contributions
RZ and XT contributed equally to the conception and design of the study. RZ and ZM wrote the manuscript. All authors contributed to the diagnosis and treatment of the case. All authors read, approved the submitted version, and agreed to be accountable for all aspects of the research in ensuring the accuracy of this study. All authors have given consent to the publication of this manuscript.
Funding
This study was funded by the National Natural Science Foundation of China grants (#81571709 and #81971650 to ZM), the Key Project of Tianjin Science and Technology Committee Foundation grant (#16JCZDJC34300 to ZM), Tianjin Medical University General Hospital New Century Excellent Talent Program (to ZM), Young and Middle-aged Innovative Talent Training Program from Tianjin Education Committee (to ZM), and Talent Fostering Program (the 131 Project) from Tianjin Education Committee and Tianjin Human Resources and Social Security Bureau (to ZM).
Acknowledgments
The authors thank Dr. Qiang Jia, who has always been a source of encouragement and inspiration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2022.1610221/full#supplementary-material
Supplementary Figure S1HE staining of samples taken from the peritoneal nodules (original magnification × 400).
Supplementary Figure S2Search criteria of eligible articles and cases.
Supplementary Table S1Timeline of laboratory tests. CA125, Carbohydrate Antigen-125; Tg, thyroglobulin; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Supplementary Table S2Patient’s clinicopathologic parameters. CK19, cytokeratin-19; TTF-1, thyroid transcription factor-1.
Abbreviations
CA125, carbohydrate antigen-125; CK19, cytokeratin-19; CT, computed tomography; FT4, free thyroxine; K-W test, Kruskal-Wallis test; N/A, not applicable; RAI, radioactive iodine; SO, Struma ovarii; Tg, thyroglobulin; TSH, thyroid-stimulating hormone; TTF-1, thyroid transcription factor-1.
References
1.
Gonet A Ślusarczyk R Gąsior-Perczak D Kowalik A Kopczyński J Kowalska A . Papillary Thyroid Cancer in a Struma Ovarii in a 17-Year-Old Nulliparous Patient: A Case Report. Diagnostics (2020) 10(1):45. 10.3390/diagnostics10010045
2.
Fujiwara S Tsuyoshi H Nishimura T Takahashi N Yoshida Y . Precise Preoperative Diagnosis of Struma Ovarii with Pseudo-meigs' Syndrome Mimicking Ovarian Cancer with the Combination of 131I Scintigraphy and 18F-FDG PET: Case Report and Review of the Literature. J Ovarian Res (2018) 11(1):11. 10.1186/s13048-018-0383-2
3.
Jean S Tanyi JL Montone K McGrath C Lage-Alvarez MM Chu CS . Papillary Thyroid Cancer Arising in Struma Ovarii. J Obstet Gynaecol (2012) 32(3):222–6. 10.3109/01443615.2011.645921
4.
Goffredo P Sawka AM Pura J Adam MA Roman SA Sosa JA . Malignant Struma Ovarii: A Population-Level Analysis of a Large Series of 68 Patients. Thyroid (2015) 25(2):211–5. 10.1089/thy.2014.0328
5.
Dardik RB Dardik M Westra W Montz FJ . Malignant Struma Ovarii: Two Case Reports and a Review of the Literature. Gynecol Oncol (1999) 73(3):447–51. 10.1006/gyno.1999.5355
6.
Makani S Kim W Gaba AR . Struma Ovarii with a Focus of Papillary Thyroid Cancer: A Case Report and Review of the Literature. Gynecol Oncol (2004) 94(3):835–9. 10.1016/j.ygyno.2004.06.003
7.
Wang L Chen X Liu B . Lower Lip Teratoma with Ventral Capillary Malformation in an Infant: Case Report and Literature Review. Int J Oral Maxill Surg (2009) 38(12):1330–3. 10.1016/j.ijom.2009.06.018
8.
Outwater EK Siegelman ES Hunt JL . Ovarian Teratomas: Tumor Types and Imaging Characteristics. RadioGraphics (2001) 21(2):475–90. 10.1148/radiographics.21.2.g01mr09475
9.
Stanojevic B Dzodic R Saenko V Milovanovic Z Krstevski V Radlovic P et al Unilateral Follicular Variant of Papillary Thyroid Carcinoma with Unique KRAS Mutation in Struma Ovarii in Bilateral Ovarian Teratoma: A Rare Case Report. BMC Cancer (2012) 12:224. 10.1186/1471-2407-12-224
10.
Coyne C Nikiforov YE . RAS Mutation-Positive Follicular Variant of Papillary Thyroid Carcinoma Arising in a Struma Ovarii. Endocr Pathol (2010) 21(2):144–7. 10.1007/s12022-009-9097-8
11.
Kabukcuoglu F Baksu A Yilmaz B Aktumen A Evren I . Malignant Struma Ovarii. Pathol Oncol Res (2002) 8(2):145–7. 10.1007/bf03033726
12.
Rosenblum NG LiVolsi VA Edmonds PR Mikuta JJ . Malignant Struma Ovarii. Gynecol Oncol (1989) 32(2):224–7. 10.1016/s0090-8258(89)80037-x
13.
Volpi E Ferrero A Nasi PG Sismondi P . Malignant Struma Ovarii: a Case Report of Laparoscopic Management. Gynecol Oncol (2003) 90(1):191–4. 10.1016/s0090-8258(03)00142-2
14.
Shrimali RK Shaikh G Reed NS . Malignant Struma Ovarii: The West of Scotland Experience and Review of Literature with Focus on Postoperative Management. J Med Imaging Radiat Oncol (2012) 56(4):478–82. 10.1111/j.1754-9485.2012.02394.x
15.
Devaney K Snyder R Morris HJ Tavassoli FA . Proliferative and Histologically Malignant Struma Ovarii. Int J Gynecol Pathol (1993) 12(4):333–43. 10.1097/00004347-199310000-00008
16.
Boutross-Tadross O Saleh R Asa SL . Follicular Variant Papillary Thyroid Carcinoma Arising in Struma Ovarii. Endocr Pathol (2007) 18(3):182–6. 10.1007/s12022-007-0022-8
17.
Pardo-Mindan FJ Vazquez JJ . Malignant Struma Ovarii: Light and Electron Microscopic Study. Cancer (1983) 51(2):337–43. 10.1002/1097-0142(19830115)51:2<337::aid-cncr2820510229>3.0.co;2-5
18.
Shaco-Levy R Bean SM Bentley RC Robboy SJ . Natural History of Biologically Malignant Struma Ovarii: Analysis of 27 Cases with Extraovarian Spread. Int J Gynecol Pathol (2010) 29(3):212–27. 10.1097/PGP.0b013e3181bfb133
19.
Shaco-Levy R Peng RY Snyder MJ Osmond GW Veras E Bean SM et al Malignant Struma Ovarii: A Blinded Study of 86 Cases Assessing Which Histologic Features Correlate with Aggressive Clinical Behavior. Arch Pathol Lab Med (2012) 136(2):172–8. 10.5858/arpa.2011-0092-OA
20.
Zhang X Axiotis C . Thyroid-type Carcinoma of Struma Ovarii. Arch Pathol Lab Med (2010) 134(5):786–91. 10.5858/134.5.786
21.
Wei S Baloch ZW LiVolsi VA . Pathology of Struma Ovarii: A Report of 96 Cases. Endocr Pathol (2015) 26(4):342–8. 10.1007/s12022-015-9396-1
22.
Yoo S-C Chang K-H Lyu M-O Chang S-J Ryu H-S Kim H-S . Clinical Characteristics of Struma Ovarii. J Gynecol Oncol (2008) 19(2):135–8. 10.3802/jgo.2008.19.2.135
23.
Hatami M Breining D Owers RL Del Priore G Goldberg GL . Malignant Struma Ovarii - A Case Report and Review of the Literature. Gynecol Obstet Invest (2008) 65(2):104–7. 10.1159/000108654
24.
Zakhem A Aftimos G Kreidy R Salem P . Malignant Struma Ovarii: Report of Two Cases and Selected Review of the Literature. J Surg Oncol (1990) 43(1):61–5. 10.1002/jso.2930430116
25.
Robboy SJ Scully RE . Strumal Carcinoid of the Ovary: An Analysis of 50 Cases of a Distinctive Tumor Composed of Thyroid Tissue and Carcinoid. Cancer (1980) 46(9):2019–34. 10.1002/1097-0142(19801101)46:9<2019::aid-cncr2820460921>3.0.co;2-w
26.
Lazarus JH Richards AR MacPherson MJ Dinnen JS Williams ED Owen GM et al Struma Ovarii: A Case Report. Clin Endocrinol (1987) 27(6):715–20. 10.1111/j.1365-2265.1987.tb02955.x
27.
Anastasilakis AD Ruggeri R-M Polyzos SA Makras P Molyva D Campennì A et al Coexistence of Graves' Disease, Papillary Thyroid Carcinoma and Unilateral Benign Struma Ovarii: Case Report and Review of the Literature. Metabolism (2013) 62(10):1350–6. 10.1016/j.metabol.2013.05.013
28.
Huh JJ Montz FJ Bristow RE . Struma Ovarii Associated with Pseudo-Meigs' Syndrome and Elevated Serum CA 125. Gynecol Oncol (2002) 86(2):231–4. 10.1006/gyno.2002.6741
29.
Yanaranop M Tiyayon J Siricharoenthai S Nakrangsee S Thinkhamrop B . Rajavithi-ovarian Cancer Predictive Score (R-OPS): A New Scoring System for Predicting Ovarian Malignancy in Women Presenting with a Pelvic Mass. Gynecol Oncol (2016) 141(3):479–84. 10.1016/j.ygyno.2016.03.019
30.
Elisei R Romei C Castagna MG Lisi S Vivaldi A Faviana P et al RET/PTC3 Rearrangement and Thyroid Differentiation Gene Analysis in a Struma Ovarii Fortuitously Revealed by Elevated Serum Thyroglobulin Concentration. Thyroid (2005) 15(12):1355–61. 10.1089/thy.2005.15.1355
31.
Kraemer B Grischke E-M Staebler A Hirides P Rothmund R . Laparoscopic Excision of Malignant Struma Ovarii and 1 Year Follow-Up without Further Treatment. Fertil sterility (2011) 95(6):2124. 10.1016/j.fertnstert.2010.12.047
32.
Ukita M Nakai H Kotani Y Tobiume T Koike E Tsuji I et al Long-term Survival in Metastatic Malignant Struma Ovarii Treated with Oral Chemotherapy: A Case Report. Oncol Lett (2014) 8(6):2458–62. 10.3892/ol.2014.2587
33.
Marti JL Clark VE Harper H Chhieng DC Sosa JA Roman SA . Optimal Surgical Management of Well-Differentiated Thyroid Cancer Arising in Struma Ovarii: A Series of 4 Patients and a Review of 53 Reported Cases. Thyroid (2012) 22(4):400–6. 10.1089/thy.2011.0162
Summary
Keywords
case report, surgery, struma ovarii, hyperthyroidism, CA125, thyroglobulin
Citation
Zhang R, Tian X, Luo Y, Dong H, Tian W, Zhang Y, Li D, Sun H and Meng Z (2022) Case Report: Recurrent Malignant Struma Ovarii With Hyperthyroidism and Metastases, A Rare Case Report and Review of the Literature. Pathol. Oncol. Res. 28:1610221. doi: 10.3389/pore.2022.1610221
Received
27 November 2021
Accepted
21 April 2022
Published
10 May 2022
Volume
28 - 2022
Edited by
Anna Sebestyén, Semmelweis University, Hungary
Updates
Copyright
© 2022 Zhang, Tian, Luo, Dong, Tian, Zhang, Li, Sun and Meng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaowei Meng, zmeng@tmu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.